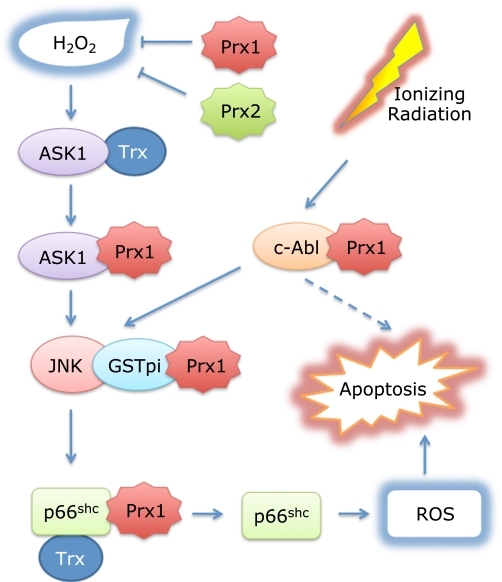
Fig. 1.
Multiple roles of Prx1 in suppression of oxidative stress-induced apoptosis. In addition to the Trx peroxidase activity to eliminate H2O2, Prx1 cooperates with Trx in suppression of H2O2-induced activation of the two apoptosis signal regulators, ASK1(26) and p66Shc,(27) through direct interactions. The activation of ASK1 by oxidant stress is inhibited by reduced form of Trx under low stress conditions. When human embryonic kidney 293 cells were treated with 5 mM H2O2 for 20 min, oxidized Trx was replaced by oxidized Prx1 to further suppress ASK1 activation.(26) Under low stress conditions, Trx and Prx1 maintain p66Shc inactive, by keeping it reduced and dimeric form. Under high stress conditions (1.47 mM H2O2), Prx1 is oxidized forming a decameric chaperone and separated from p66Shc, which is phosphorylated at Ser-36. JNK phosphorylates p66Shc at Ser-36,(38,39) inducing tetramer formation and translocation of p66Shc to mitochondria to generate ROS.(27,37) On the other hand, ionizing radiation activates tyrosine kinase c-Abl(41) and JNK.(28) We speculate Prx1 may inhibit activation of c-Abl following ioning radiation. Prx1 indirectly binds JNK through GSTpi, which suppress activation of JNK.(28) Prx2 like Prx1 suppresses ASK1 activation in neuronal cells in which Prx2 is highly expressed.(29)