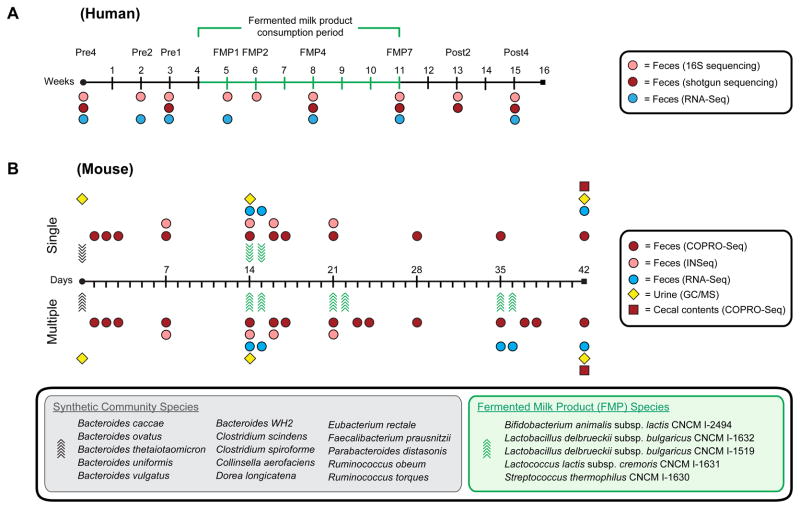
Figure 1. Experimental design for human and mouse studies.
(A) Human study. Seven healthy lean MZ female twin pairs were sampled before, during, and after FMP consumption. (B) Gnotobiotic mouse study. Two groups of five germ-free mice were colonized by oral gavage at 6–8 weeks of age with a 15-member microbial consortium constituting a model human gut microbiota (day of gavage denoted by black arrows). Two weeks later, the five species FMP strain consortium was administered by oral gavage to each group of mice twice over two days (denoted by green arrows). Mice in the single treatment group underwent no further manipulations, whereas animals in the multiple treatment group received additional two-day gavages one and three weeks following the first gavage. Samples were collected at the indicated time points for profiling bacterial community membership (shotgun and 16S rRNA gene sequencing for human fecal samples, COPRO-Seq for mouse fecal and cecal samples), gene expression profiling (microbial RNA-Seq) and metabolite analysis (urines, GC/MS). The species comprising the model 15-member human community and the 5-member FMP consortium are listed in the gray and green boxes, respectively.