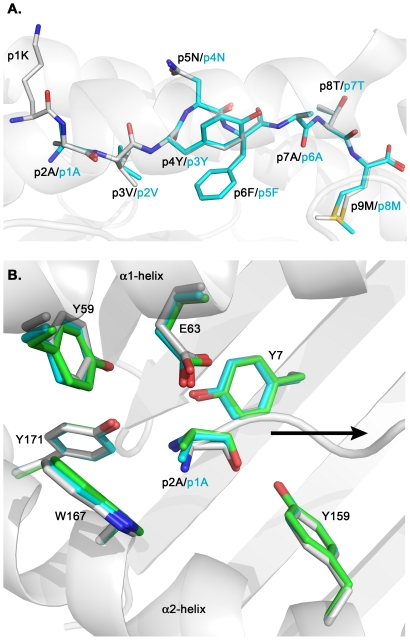
Figure 2. Protrusion of p1K in H-2Kb/gp33 does not affect the overall conformation of the N-terminal part of the peptide binding cleft and conserves the conformation of the epitopes.
A. Superimposed side views of the peptides gp33 (KAVYNFATM) and gp34 (AVYNFATM) depicting how residue p1K in gp33 protrudes out of the peptide binding cleft of H-2Kb. The remaining residues of gp33 take a similar conformation to all the side chains of gp34. The peptides gp33 and gp34, annotated in black and cyan, respectively, are depicted with their N termini to the left and their C termini to the right. The carbon atoms of the peptides gp33 and gp34 are colored in white and cyan, respectively. Carbon, nitrogen and oxygen atoms are in cyan, blue and red, respectively. The peptide-binding cleft of H-2Kb is colored white. B. Conformation of side chain residues interacting with the N-termini of peptides in the crystal structures of H-2Kb/gp33 (in white) and H-2Kb/gp34 (both MHC complexes from the asymmetric unit are displayed in cyan and light green, respectively), following superposition of the α1α2 domains. Note that the p2A residue in gp33 occupies the position corresponding to the p1A in gp34. The side chain of p1K in gp33 is not displayed. The orientation of the peptides is depicted by a black arrow (from the N terminus toward the C terminus). The α1 and α2 helices are indicated.