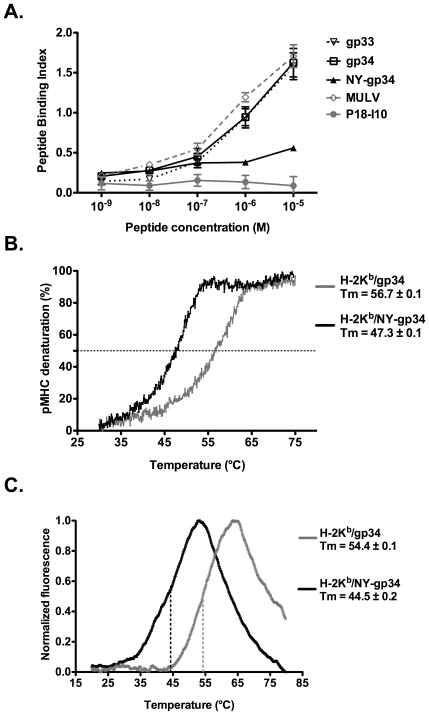
Figure 4. Nitrotyrosination of gp34 significantly decreases both its binding affinity and stabilization capacity of the H-2Kb complex.
A. The binding affinity of NY-gp34to H-2Kbon the surface of TAP-deficient RMA-S cells was significantly reduced when compared to gp33 and gp34. Cells were incubated with the indicated concentrations of each peptide at 26°C for 12 hours in 5% CO2. Cells were collected and stained with the H-2Kb-specific antibody AF6-88.5. The mean fluorescence intensities (MFI) observed for each timepoint were normalized as described in the peptide binding affinity assays outlined above. The H-2Dd and H-2Db-restricted peptides P18-I10 and MulV were used as negative and positive controls, respectively. The peptide binding assays were repeated three times. B. The capacity of NY-gp34 to stabilize soluble H-2Kb complexes is significantly reduced when compared to gp34. MHC complex melting temperatures (Tm) were measured using circular dichroism. Tm values were derived from normalized thermal denaturation curves for the MHC complexes H-2Kb/gp34 (in grey) and H-2Kb/NY-gp34 (in black) as the temperature corresponding to 50% denaturation (dashed line). The presented denaturation curves are an average from at least two independent measurements for each MHC complex. C. The denaturation curves of the two MHC complexes, using the differential scanning fluorimetry approach (thermofluor), are consistent with the two-state model of thermal unfolding. The melting temperatures, defined as the inflection points of the escalating parts of the curves, the curves of H-2Kb/gp34 and H-2Kb/NY-gp34 are in grey and black, respectively.