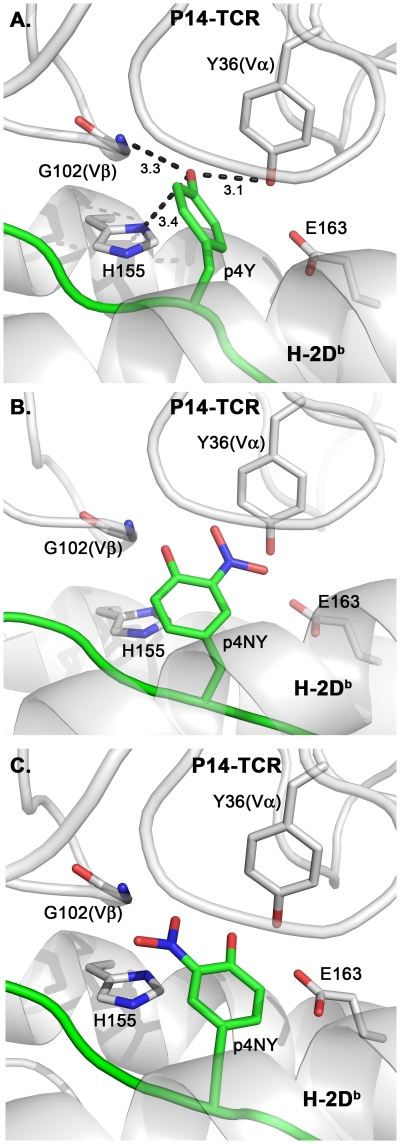
Figure 5. Nitrotyrosination of p4Y in H-2Db/NY-gp33 directly affects recognition by H-2Db/gp33-specific TCR.
Nitrotyrosination of the main TCR-interacting peptide residue p4Y will affect the structural conformation of both TCR interacting residues on H-2Db and of the TCR P14. The peptide binding cleft of H-2Db and the TCR, both colored in white, are annotated. Hydrogen bond interactions appear as dotted lines. A. In H-2Db/gp33, the side chain of p4Y protrudes out of the H-2Dbpeptide-binding cleft, positioning itself perfectly in the hot spot of the p14 TCR composed of the CDR3 loops from both Vα and Vβ. It forms three hydrogen bonds, two of them directly with Y36(Vα) and G102(Vβ) on the TCR P14. The last hydrogen bond is formed with the side chain of the H-2Db histidine residue H155, linking this domain of the heavy chain to the TCR. B. The side chain of the nitrotyrosinated p4-NY can not be accommodated within the hot-spot of P14, resulting in sterical clashes with the side chain of the TCR residue Y36(Vα). Furthermore, the negatively charged side chain of the H-2Db residue E163, important for TCR recognition, would also be repelled by the introduced negatively charged nitrotyrosination. C. Similarly, the other rotamer of the nitrotyrosinated p4-NY would result in sterical clashes with both G102(Vβ)the side chain of H155, abolishing all formed hydrogen bond interactions.