Figure 7.
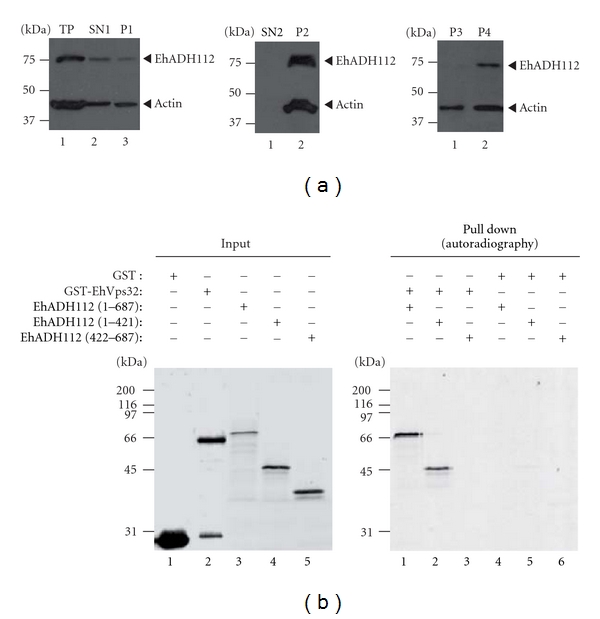
EhADH112 major presence in membrane subcellular fractions and EhADH112 interaction with EhVps32. (a) Location of EhADH112 in wild-type trophozoites subcellular fractions. Proteins (50 μg) from different fractions of trophozoite extracts were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. Western blot assays were performed using pαEhADH243 and mαactin antibodies and corresponding peroxidase-labeled anti-rabbit or anti-mouse IgG secondary antibodies. Membranes were revealed by chemiluminescence. Left: SN1 and P1 fractions resulting after total proteins (TP) centrifugation at 250 ×g for 30 min in a mannitol/sucrose gradient. Middle: SN2 and P2 fractions resulting after SN1 ultracentrifugation at 40 000 ×g for 60 min. Right: P3 and P4 fractions obtained after ultracentrifugation of solubilized P1 at 40 000 ×g for 60 min. (b) Binding of EhADH112 to EhVps32 through the Bro1 domain. GST or GST-EhVps32 proteins were immobilized on glutathione-Sepharose beads and incubated with in vitro synthesized [35S]-EhADH112 or [35S]-EhADH112 derivatives. Left: purified GST and GST-EhVps32 fusion proteins or [35S]-EhADH112 and [35S]-truncated derivatives (autoradiography, lanes 3, 4 and 5) used for binding experiments (8% of the total reaction mixture). Right: pulled-down proteins electrophoresed on 10% SDS-polyacrylamide and detected by autoradiography.
