Abstract
In the spinal cord, neurons and glial cells actively interact and contribute to neurofunction. Surprisingly, both cell types have similar receptors, transporters and ion channels and also produce similar neurotransmitters and cytokines. The neuroanatomical and neurochemical similarities work synergistically to maintain physiological homeostasis in the normal spinal cord. However, in trauma or disease states, spinal glia become activated, dorsal horn neurons become hyperexcitable contributing to sensitized neuronal-glial circuits. The maladaptive spinal circuits directly affect synaptic excitability, including activation of intracellular downstream cascades that result in enhanced evoked and spontaneous activity in dorsal horn neurons with the result that abnormal pain syndromes develop.
Recent literature reported that spinal cord injury produces glial activation in the dorsal horn; however, the majority of glial activation studies after SCI have focused on transient and/or acute time points, from a few hours to one month, and peri-lesion sites, a few millimeters rostral and caudal to the lesion site. In addition, thoracic spinal cord injury produces activation of astrocytes and microglia that contributes to dorsal horn neuronal hyperexcitability and central neuropathic pain in above-level, at-level and below-level segments remote from the lesion in the spinal cord. The cellular and molecular events of glial activation are not a simple event, rather it is the consequence of a combination of several neurochemical and neurophysiological changes following SCI. The ionic imbalances, neuroinflammation and alterations of cell cycle proteins after SCI are predominant components for neuroanatomical and neurochemical changes that result in glial activation. More importantly, SCI induced release of glutamate, proinfloammatory cytokines, ATP, reactive oxygen species (ROS) and neurotrophic factors trigger activation of postsynaptic neurons and glial cells via their own receptors and channels that, in turn, contribute to neuronal-neuronal and neuronal-glial interaction as well as microglia-astrocytic interactions. However, a systematic review of temporal and spatial glial activation following SCI has not been done.
In this review, we describe time and regional dependence of glial activation and describe activation mechanisms in various SCI models in rats. These data are placed in the broader context of glial activation mechanisms and chronic pain states. Our work in the context of work by others in SCI models demonstrate that dysfunctional glia, a condition called “gliopathy”, are key contributors in the underlying cellular mechanisms contributing to neuropathic pain.
Keywords: Central Neuropathic Pain, Glial Activation, Gliopathy, Hyperexcitability, Spinal Cord Injury
The lack of systematic studies in glial activation after SCI
In the spinal dorsal horn, neurons and glial cells interactively contribute to spinal neurofunction and neuroarchitecture, as well as the maintenance of physiological homeostasis. Neuroanatomically, neurons and glial cells express similar receptors and ion channels, but the major difference in the electrophysiological aspects between the two cell types is the neurons’ ability for generation and propagation of action potentials in the nervous system (Eulenburg and Gomeza, 2010; Jarvis, 2010; Porter and McCarthy, 1997). Once action potentials are developed, propagation to supraspinal nervous system structures can occur via synapse-synapse interaction between presynaptic and postsynaptic neurons. Interestingly, glial cells, once thought to be rather inert, participate actively in local synaptic transmission (Takahashi and Tsuruhara, 1987; Yang et al., 2009), but do not generate and propagate action potentials. Due in large part to the differential neuroanatomical and electrophysiological membrane properties, glial cells were thought not to directly participate in sensory physiology, more specifically in pain transmission mechanism. Gial cells, ie. astrocytes and microglia, were thought to participate in maintaining homeostasis via transmitter and ion uptake, to regulate extracellular neurotransmitter concentration, pH and ionic balance, and neuroinflammation in both normal physiological and pathophysiological conditions in the nervous system (Jendelová and Syková, 1991; Korn et al., 2005; Syková and Chvátal, 1993).
One methodological in vitro tool to detect astrocytic and microglial cell types is the use of cell specific antibody-antigens reactions. In the spinal cord, specific surface markers to detect glial cells have been identified, such as CD11b, CD18, Mac-1, ITGAM, CD14, CD44, MHC I and II. However, ALDH1L1 and anti-glial fibrillary acid protein (anti-GFAP) reaction products have been used to visualize astrocytes whereas CD68, Iba1 and OX-42 antibody (which recognizes the CD11b antigen) are useful immunoreactive products to visualize microglia (Jacque et al., 1978; Graeber et al., 1989; Watanabe et al., 1999). Additionally, astrocytic and microglial activation is characterized by somatic hypertrophy (increased cell volume), thickened and ramified branches, and proliferation. In vitro experiments demonstrate that increased release of proinflammatory cytokines (such as interleukin-1β and tumor necrosis factorα) and chemokines (such as CXCL2 and CCL2) are useful bioassay products to detect both astrocytic and microglial activation (Tzeng et al., 1999). Glial (astrocytes and microglia) cells are easily activated by chemical and mechanical injuries to the spinal cord, such as trauma, inflammation, ischemia, radiation and excitoxicity (Fitch et al., 1999; Kyrkanides et al., 1999; Tikka et al., 2001). Activated glial cells produce abnormally increased secretory products and contribute to alterations in uptake mechanisms and reversal of transporter systems that suggest glial cells no longer maintain homeostasis but contribute to spinal circuit dysfunction. Thus activated glial cells are able to influence maladaptive neurophysiological and neuroanatomical changes in synaptic circuits and that lead to abnormal sensory transmission. This is a condition we term gliopathy (Gwak and Hulsebosch, 2010b; Hulsebsoch, 2008).
For over a decade, however, new concepts of synaptic signaling mediated by activated glial cells have emerged. Neurons and glial cells express similar receptors, ion channels and transporters as well as have similar intracellular signaling cascades for activation. Glial cells also actively communicate with neighboring neurons via tight junctions (Nedergaard, 1994; Roh et al., 2010; Zündorf et al., 2007) and synapses (Haber et al., 2006; Oliet et al., 2008). In addition, it is well documented that neurotrauma, such as spinal cord injury (SCI) and peripheral nerve injury (PNI), produces physiological and morphological activation of glial cells, specifically in astrocytes and microglia (Colburn et al., 1999; Fujiki et al., 1996; Lee et al., 2000; Tzeng et al., 1999). Taken together, neuroanatomical and neurochemical changes derived from activated glial cells are important contributors to the altered sensory transmission in the spinal dorsal horn following neurotrauma. Recent review articles document spatial and temporal glial activation in peripheral nerve injury models (Austin and Moalem-Taylor, 2010). For example, after peripheral nerve injury, microglia are activated within 24 hours after injury in the spinal dorsal horn whereas astrocytes are activated 3 days after injury. Astrocytic and microglial activation lasted for as long as 3 months in the dorsal horn after injury and then subsides. The activation of astrocytes and microglia correlates well with neuropathic pain behaviour, such as mechanical allodynia and thermal hyperalgesia, following peripheral nerve injury (Coyle, 1998; Ledeboer et al., 2005; Zhuang et al., 2005). However, the majority of glial activation studies after SCI in mammalian models, including our own work, have focused on transient and/or acute time points, from a few hours to one month, and only examined peri-lesion sites, a few millimeters rostral and caudal to the lesion site, and neglected chronic (over a month) and remote regions several spinal segments rostral and caudal to the injury site (see the section of Glial Activation in SCI Models). Recently, our laboratory reported that activation of astrocytes and microglia contributes to neuronal hyperexcitability in spinal dorsal horn neurons, and thus central neuropathic pain behavior that lasts over a month, in the forepaws (above-level), the body trunk (at-level) and the hindpaws (below-level) following SCI (Carlton et al., 2009, Crown et al., 2008; Gwak et al., 2008; Gwak and Hulsebosch, 2009). Collective previous reports, including our reports, propose that spatial and temporal activation of glial cells, specifically astrocytes and microglia, following SCI are critical to the maintained (over a month) dorsal horn neuronal hyperexcitability; however, mechanisms that can account for all three anatomic regions of central sensitization are not well described.
Because no evidence exists that report the systematic spatial and temporal activation of astrocytes and microglia in the dorsal horn at immediate, acute, subacute and chronic time points, we present data that is consistent with activation of astrocytes and microglia at three different regions of the spinal dorsal horn following low thoracic contusion injury by detecting increases of specific immunoreactive products that characterize activation of astrocytes and microglia, respectively.
Spatial and Temporal Glial Activation in the Spinal Dorsal Horn following Contusive Spinal Cord Injury
Spinal Cord Injury
Male Sprauge-Dewley rats (225-250 g, n=84) were used in this study. Moderate T10 spinal contusion injury (150 kdyne 1 sec dwell time, n=42) was performed using Infinity Horizon Impactor (IH Impactor). Briefly, rats were anesthezied by intraperitoneal (i.p.) injection of sodium pentobarbital (60 mg/kg), the back of thoracic regions shaved and topical iodine applied. The skin was incised, the musculature was retracted and after identification of T7/8 vertebrae, a bilateral laminectomy was done to expose the spinal T10 region. Rats were mounted at IH impactor frame and contused by computer controlled device impactor (150 kdyne, 1 sec dwell). After contusion, the musculature was sutured and the skin was autoclipped. Rats were placed on a heating pad until recovery from anesthesia, usually within 2 hrs. As a control group, sham surgery (n=42) was performed with the same procedures with the exception that no spinal contusion injury was performed. Rats were housed (3 rats per cage) in reversed 12h day/12h night cycles and fed Ad libitum. All experimental procedures were reviewed by the UTMB Institutional Animal Care and Use Committee (IACUC) and are consistent with the guidelines of the International Association for the Study of Pain and the NIH Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry
The spatial and the temporal activation of astrocytes and microglia was examined by measuring the immunoreaction production (intensity) of GFAP (for astrocytes) and OX-42 (for microglia) in three different regions of spinal cord; above-level (cervical regions, C6/7), at-level (thoracic regions, T7/8) and below-level (lumbar regions, L4/5) at 2 hrs following surgery and post operation days (POD), 1, 7, 14, 30, 90 and 180 at both SCI and age-matched sham control groups (n=6, each time point), respectively. Briefly, rats were anesthetized by sodium pentobarbital (i.p. 100 mg/kg) and heparin contained saline (400 ml) intracardially perfused to flush body blood, followed by perfusion with 4% paraformaldehyde fixative (400 ml). The C6/7, T7/8 and L4/5 spinal cord was dissected en bloc and underwent post-fixation with same fixative (overnight in cold room), respectively. Each spinal cord was transferred into 30% sucrose/4% paraformaldehyde fixative and incubated at 4°C for several days for cryoprotection. The spinal cord was cryomolded using specimen mold plate (Tissue-Tek) individually and kept in the deep freezer (-80 °C). Spinal cords were sectioned at 20 μm thickness using a sliding microtome and were collected (eight sections per one spinal cord) by free floating methods in 0.1M phosphate buffer (PB, pH 7.4). Tissues were blocked in 5% normal goat serum with 0.1% Triton-X 100 (which allows better antibody penetration) for 45 minutes and incubated overnight in anti-mouse GFAP (for astrocytes, 1:500, Chemicon) or anti-mouse OX-42 (for microglia, 1:100, Serotec), respectively. The tissues were then rinsed (10 mins, 4 times with 0.1 M PB buffer) and incubated (2 hrs) in goat anti-mouse Alexa Fluor 488 (1:600, Molecular Probes). After rinsing (10 mins, 4 times with 0.1 M PB buffer), the floating sections were mounted on gelatin-coated slides and coverslipped with mounting medium, a non-fade media (H-1200, Vector Lab. Inc). The intensity of GFAP and OX-42 was quantified in the spinal dorsal horn (lamina I-V) at above-level (C6/7), at-level (T7/8) and below-level (L4/5) in both sham and SCI rats using Confocal Microscopy and Image Analyses software as follows. The immunoreaction product (intensity) for GFAP and OX-42 was measured using Bio-Rad Radiance Confocal Laser System coupled to a Nikon E800 camera with Lasersharp 2000 imaging software. The immunoreaction product of GFAP and OX-42 were collected with Krypton lasers of 488 nm excitation (green emission, a result of excitation of the conjugated AlexaFluor 488 antibody) that identify localization of immunoreaction product in astrocytes and microglia, respectively. To decrease background activity, the offset (control black level), gain (control brightness), the laser (control intensity) and the iris (control aperture) were properly adjusted using the Lasersharp 2000 imaging software. We have choose 2 sections (the first and the fourth sections) and the intensities of GFAP and OX-42 in the dorsal horn were evaluated using a computer-assisted image analysis program (Metamorph 6.1). The comparison of GFAP and OX-42 intensity was tested by repeated Two-way ANOVA (with two independent variables : 7 time points and 2 groups) using SigmaStat (ver 3.01). Data are displayed as intensity after normalization (to 100% of dorsal horn intensity in sham controls). The alpha level of 0.05 was chosen and data are displayed as means ± standard error (S.E.) of the mean.
Astrocytic Activation
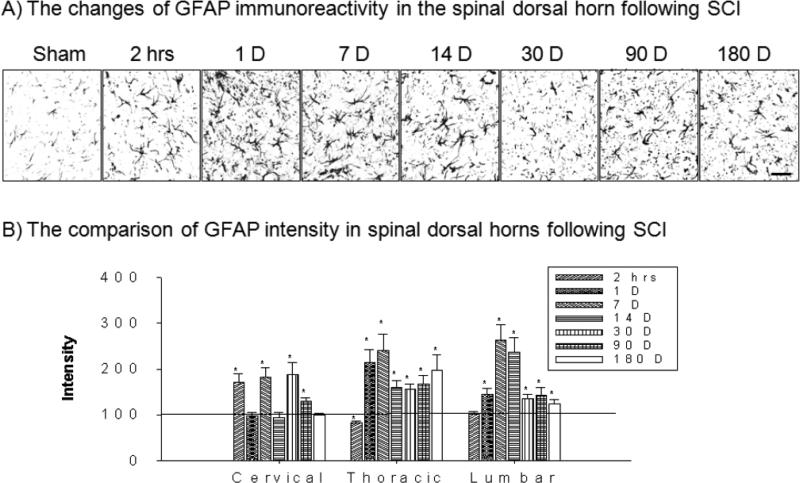
Immunoproducts of GFAP in the spinal dorsal horn demonstrated temporally increased astrocytic activation following T10 spinal cord injury (Figure 1A). In cervical 6/7 regions, the intensity of GFAP significantly increased at 2 hrs and POD 7, 30 and 90 after T10 spinal contusion injury (*p>0.05, Figure 1B). In thoracic 7/8 regions, the intensity of GFAP significantly increased at POD 1 to 180 whereas the intensity at 2 hours showed decrease (*p>0.05, Figure 1B). In lumbar 4/5 regions, the intensity of significantly increased at POD 1 to 180 whereas the intensity at 2 hour did not show significant change (*p>0.05, Figure 1B). Taken together, T10 spinal contusion injury produces the consistent astrocytic activation in the entire spinal dorsal horn.
Figure 1.
The spatial and temporal activation of astrocytes in the spinal dorsal horn
A) Examples of GFAP immunoreactions products at various time points in the lumbar spinal dorsal horn following T10 contusion injury. B) After normalization, the intensity of GFAP significantly increased immediately in cervical spinal segments whereas thoracic spinal segments showed a decrease. The GFAP intensity in lumbar spinal segments did not show significant changes. However, the intensity of GFAP from 1 day to 180 days after SCI showed consistent increases at all three different spinal regions in the dorsal horn. Scale bar : 30 μm.
Microglial Activation
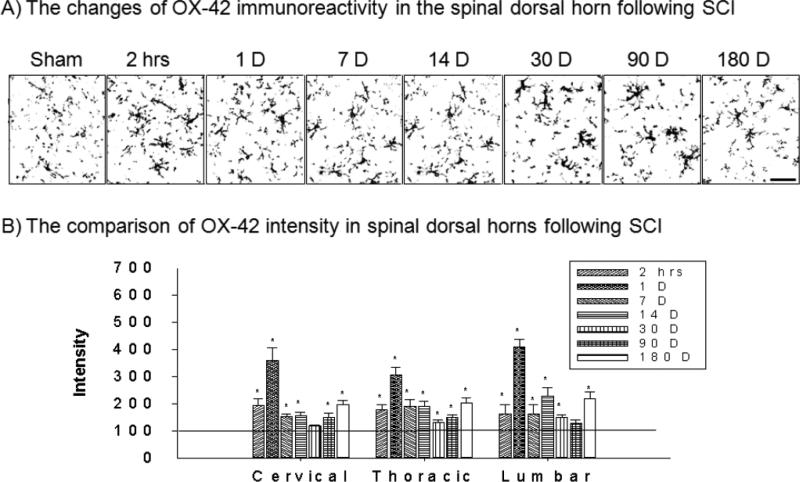
Immunoproducts of OX-42 in the spinal dorsal horn demonstrated temporally increased microglial activation following T10 spinal cord injury (Figure 2A). In cervical 6/7 regions, the intensity of OX-42 significantly increased at 2 hrs to POD 180 except for at POD 30 time point after T10 spinal contusion injury (*p>0.05, Figure 2B). In thoracic 7/8 regions, the intensity of OX-42 significantly increased at all test periods (*p>0.05, Figure 2B). In lumbar 4/5 regions, the intensity of OX-42 significantly increased at 2 hrs to POD 180 except at POD 90 time point (*p>0.05, Figure 2B). In all three different spinal regions, POD 1 showed the highest microglial activation after injury.
Figure 2.
The spatial and temporal activation of miroglia in the spinal dorsal horn
A) Examples of OX-42 immunoreactions product at various time points in the lumbar spinal dorsal horn following T10 contusion injury. B) After normalization, the intensity of OX-42 significantly increased immediately (2 hours) and lasted up to 180 days after SCI at all three different spinal regions in the dorsal horn. Scale bar : 20 μm.
In the present study, thoracic 10 contusive SCI produced glial activation of both astrocytes and microglia, characterized by somatic hypertrophy and thickened branches, in the spinal dorsal horns throughout the spinal axis; cervical (C6/7, above-level), thoracic (T7/8, at-level) and lumbar (L4/5, below-level) regions. Astrocytic and microglial activation developed immediately after injury (2 hrs after SCI) and persists chronically (6 months after SCI), suggesting persistent glial activation following SCI. Microglia demonstrated a more rapid activation than astrocytic activation in both at-level and below-level spinal regions; whereas above-level spinal regions did not show any significant difference. This is the first report that low thoracic SCI produces spatially persistent glial activation in the dorsal horns of the entire spinal axis.
Glial Activation in SCI Models
The documentation of systematic spatial and temporal glial activation in the dorsal horn is controversial in rodent SCI models. Indeed, a variety of SCI parameters that influence the injury response exist, such as age, strain, severity and region site. The literature reports a variety of astrocytic and microglial activation patterns in the spinal dorsal horn that appear to be dependent on the kind of SCI models used to evaluate the spinal dorsal horn. Currently, five rodent SCI models are predominantly used to study glial activation in the spinal dorsal horn after SCI, which is followed by sensory and motor abnormalities. We and others have reported that SCI directly and indirectly causes glial activation at both the early (within 3 days) and the chronic (at least over a month) phases of injury in rats (Gwak and Hulsebosch, 2009; Knerlich-Lukoschus et al., 2010; Nesic et al., 2005). In addition, we and others have characterized classic morphological activation markers, such as somatic hypertrophy, proliferation, thickened branches, membrane surface markers (such as CD11b, toll-like receptors and glial fibrillary acidic protein, GFAP) and physiological activation markers, such as increased release of glutamate, ROS, ATP, CGRP, interleukin-1 (IL-1), interleukin-6 (IL-6) and tumor necrosis factor (TNF) α, respectively (Chao et al., 1995; Lieberman et al., 1989; Svensson et al., 1993). A discussion of the five rodent SCI models follows;
1. Spinal Contusion Injury
Spinal contusion injury is the most relevant SCI model and best parallels the pathophysiology of human SCI. In previous reports, T10 spinal contusion injury induced by New York University injury device (NYU Impactor) resulted in activation of astrocytes near the injury site on POD 2, that decreased and returned by POD 30 (Baldwin et al., 1998) in rats. In addition, T9/10 contusion injury (NYU) in rats resulted in activation of microglia only at POD 30 several segments above (cervical) and below (low thoracic) the contusion segment; whereas, astrocytic activation in the same regions was well developed from 7 days to POD 30 (Andrade et al., 2008). However, we report that T10 contusion injury with Infinite Horizon Impactor Device (IH Impactor) produces persistent glial activation (2 hours to over 3 months) in the dorsal horn along the entire spinal axis (the current study; Nesic et al., 2005). Other studies, using a T12 contusion injury reported persistent (1 to 12 weeks) microglial activation at 5 mm rostral to the contusion region (Wu et al., 2005); whereas, spinal crush injury at T8 resulted in activated astrocytes and macrophages (which includes microglia and infiltrating macrophages) for over a month near the injured site (Fujiki et al., 1996).
2. Spinal Ischemic Injury
Photochemical SCI injury at T8 (irradiated with argon laser beam with 560 nm wavelengths after rose bengal injection) resulted in astrocytic and microglial activation on POD 2 after injury which subsided to presurgical levels by POD 12, both above (T4-5) and below (L1-2) the injured spinal region (Koshinaga and Whitemore, 1995). A different photochemical injury (8 kLux illumination with halogen lamp after bengal rose injection) also produced astrocytic activation near the injury site less than 1 hour to over 2 weeks (Verdú et al., 2003). In addition, ischemic injury to the spinal cord in rabbits also showed astrocytic and microglial activation at 2 hours after injury, which did not last chronically (over a week) (Matsumoto et al., 2003).
3. Spinal Hmisection/Hemitransection Injury
Spinal hemisection (hemitransection) at T13 produced astrocytic and microglial activation at POD 7 that persists up to 3 months in the dorsal horn throughout the spinal axis (Chadi et al., 2001; Leme and Chadi, 2001). Similarly, T12 hemisection produced microglial activation at POD 1 after injury whereas astrocytic activation developed 3 POD after injury and lasted over 4 weeks near the injury site (Tian et al., 2007). The increase of microglia activation correlated with nitric oxide synthase and peroxynitrite production (Xu et al., 1998), suggesting that glial activation is an important source of oxidative stress and neural damage following SCI. T11 transection-induced SCI rats showed a transient (3 hours to 1 day) decrease of microglia activation, whereas astrocytes did not show any significant changes, in regions 3-4 mm rostral to the SCI. However, from POD 1 to 4 weeks following SCI, astrocytic and microglial activation occured (Watanabe et al., 1999). A T8/9 spinal transection injury resulted in microglial activation at POD 2 to over 3 weeks in rats (Baloui et al., 2009). In addition, spinal hemisection at T8/9 in Macque monkeys resulted in microglial activation from 1 week to over a month in the spinal cord (Shi et al., 2009).
4. Spinal Compression Injury
After spinal cord clip compression injury at T11, astrocytic and microglial proliferation developed near the lesion site at POD 1 after injury (Jung et al., 2003; Li et al., 1995). In addition, using an electromagnetic device to induce compression injury at T8 resulted in astrocytic activation that developed at both rostral (10 mm) and caudal (10 mm) regions from POD 2 after injury to over 1 month in mice (Chen et al., 2005).
5. Spinal Excitotoxic Injury
NMDA-induced excitotoxic injury at T7 showed that astrocytic and microglial activation developed at POD 1 after injury (Gomes-Leal et al., 2004). In addition, quinolinic acid (QA, NMDA receptor agonist)-induced excitotoxic injury resulted in activation of microglia, whereas activation of astrocytes decreased at POD 1 after injury. However, astrocytic and microglial activation was well developed at POD 7 to 1 month after injury (Dihné et al., 2001).
To summarize, the collective data from different SCI model studies suggest different glial activation patterns; however, it is certain that SCI produces acute (within few days) to persistent (over a month) glial activation that is characterized by cell hypertrophy, proliferation and thickened glial branches, in the spinal dorsal horn. However, note that inter-comparison between SCI models suggests that spinal contusion injuries produce longer lasting (over a month) astrocytic and microglial activation whereas the ischemic injuries produce transient (less than 2 weeks) increases.
There have been confirmatory studies that glial activation contributes to enhanced synaptic efficacy and neuronal hyperexcitability (Gwak et al., 2008; Hains and Waxman, 2006; Popovich et al., 1997). Futhermore, increased neurotransmitters, neuropeptides, proinflammatory cytokines (such as IL-1, IL-6 and TNFα) are all strong candidates for enhancement of excitatory sensory circuits following SCI (Bennett et al., 2000; Detloff et al., 2008). Thus, activated glial-neuronal interactions directly contribute to central neuropathic pain following SCI (Gwak et al., 2008; Gwak and Hulsebosch, 2010b; Hulsebosch, 2008, see Glial Activation on Central Neuropathic Pain Following SCI, below).
Glial Activation Mechanisms Following SCI
Following SCI, cellular and molecular events occur that induce glial activation in the spinal dorsal horn. In the central nervous system, glial activation is not a simple event, rather it is the consequence of a combination of several neurochemical and neurophysiological changes following SCI. In the spinal dorsal horn, neuron and glial cells (astrocytes and microglia) are highly organized with synaptic structures that suggest interactive synaptic transmission. Those synaptic structures provide complex, rather than simple, synaptic transmission following SCI (Scholz and Woolf, 2007). The following cellular and molecular events suggest activation of glial cells in the dorsal horn following SCI.
1. Glial activation by the direct coupling between primary afferent fibers and glial cells
SCI directly triggers enhanced release of substances, such as glutamate, proinfloammatory cytokines, ATP, reactive oxygen species (ROS) and neurotrophic factors from injured primary afferent fibers at synaptic clefts that trigger activation of postsynaptic neurons via their own receptors (McMahon and Malcangio, 2009; Scholz and Woolf, 2007; Vallejo et al., 2010). Those substances that stimulate neuronal-neuronal synaptic transmission also trigger activation of neighboring astrocytes and microglia because they also express similar receptors and ion channels. For example, glutamate-GluRs (Pirttimaki et al., 2011; Weisshaar et al., 2010), substance p-NK1 receptor (Gitter et al., 1994), nitric oxide-G-protein (Willmott et al., 2000), ATP-P2Xy receptors (Pam et al., 2000), Na+-Na channels/Ca2+-Ca channels (Rose et al., 1997; Scemes and Giaume, 2006; Schimizu et al., 2007), chemokines-CX3CRs (Zhao et la., 2007a), Toll-like receptors (Bisbis et al., 2002) and interleukins (Sawada et al., 1993) are predominant receptor couplings for neuronal-glial interactions following SCI. A typical activation pathway follows; increased extracellular glutamate activates metabotropic glutamate and ionotropic glutamate receptors, such as NMDA and Kainate, receptors, followed by activation of MAPK family, such as p38 MAPK and ERK, that result in increases of pNR1 and iNOS expression in the microglia (Zheng et al., 2010; Crown et al., 2008). Then, activated microglia release IL-1β, IL-6, TNFα, ROS, BDNF, and CCL2 that triggers activation of postsynaptic neurons that lead to neuronal hyperexcitability (McMahon and Malcangio, 2009; Nakagawa and Kaneko, 2010). In addition, activated microglia facilitate calcium entry into astrocytes that trigger astrocytic calcium waves. For example, activated microglia release glutamate, proinflammatory cytokines and ROS followed by activation of NMDA and P2X1/5 receptors that result in astrocytic calcium signaling (Palygin et al., 2010). The facilitation of calcium signaling in astrocytes releases glutamate extracellularly, followed by NMDA receptor-mediated facilitation of neuronal excitability, a substrate of pain transmission (Bardoni et al., 2010).
2. Glial activation by extracellular ionic imbalances following SCI
SCI produces long lasting (over 2 weeks) upregulation of high affinity glutamate transporters in surviving astrocytes, such as GLT1, in the spinal dorsal horn; although SCI produces decreased GLT protein expression and loss of GLT expressing astrocytes (Lepore et al., 2011). Upregulation and/or reversal of glutamate transporters, such as GLTI and GLAST, facilitate uptake of extracellular glutamate and increased intracellular glutamate and contribute to astrocytic swelling, such as hypertrophy. Upregulation of GLT1 also stimulates uptake of K+ via Na+- and Ca2+-dependent mechansims as well as activation of Na+/K+–ATPase (Bender et al., 1998; Sontheimer et al., 1994). The accumulation of K+ leads to anion channel opening, enhances the passive influx of Cl-, K+, HCO -3, followed by H2O accumulation in the astrocytes that results in astrocytic hypertrophy. In addition, SCI causes decreased expression of inwardly-rectifying potassium channels 4.1 (Kir4.1) in astrocytes, the loss of Kir4.1 causes loss of K+ homeostasis and facilitates astrocytic depolarization (Olsen et al., 2010). In addition, over-production of intracellular glial ROS after SCI activates astrocytic mitogen-activated protein kinases and Na+/K+/Cl- (NKCC) co-transporters and increases intracellular uptake of K+ ions with release of Na+ ions into the extracellular space (Jayakumar et al., 2008; Moriyama et al., 2010). Thus, the K+ and Na+ ionic imbalance at glial-neuronal synaptic clefts results in the reduction of K+-mediated resting potentials and initiates Na+-mediated depolarization of neuronal membranes followed by Ca2+-mediated intracellular events that result in persistent hyperexcitability of the spinal dorsal horn neurons following SCI, the substrate for central neuropathic pain (Hulsebosch et al., 2009; Waxman and Hains, 2006).
3. Glial activation by neuroinflammation
After SCI, neurons and glial cells immediately (less than 1 hour) release proinflammatory cytokines and chemokines that contribute to proliferation/activation, migration, inflammation and primary/secondary cell damage (Choi et al., 2010; Donnelly and Popovich, 2008; Jones et al., 2002; Song et al., 2001). Those cytokines and chemokines also recruit circulating immune cells, such as leukocytes and T cells, that are involved in persistent glial activation in the spinal cord following injury (Detloff et al., 2008; Rice et al., 2007; Yang et al., 2004).
Cytokines
A cytokine is a small cell signalling protein that is released by neurons, glial cells and immune cells. Proinflammatory cytokines are cytokines that induce the inflammation response. After SCI, neuronal and glial cells release proinflammatory cytokines, such as IL-1β and TNFα, within 5 mins, followed by release of IL-6 and leukemia inhibitory factor (LIF) (Pineau and Lacroix, 2007, Nesic et al., 2005). The proinflammatory cytokine production is also detected 28 days after SCI via resident neural cells (neurons and glial cells) and infiltrating immune cells (macrophage and T lymphocytes). Acutely, increased IL-1β and TNFα is reported to be a direct result of astrogliosis and microgliosis, ie., activated glial cells. For example, IL-1β induces expression of intercellular adhesion molecule-1 (ICAM-1) and TNFα alpha induces chemokine CXCL 10 and MCP-1 (monocyte chemo-attractant protein-1, also known as CCL2 and type I inflammatory monocyte), that both contributes to astrocytic activation (Ballestas et al., 1995; Gao et al., 2010; William, et al., 2009). Thus, proinflammatory cytokines are directly involved in persistent glial activation in both the acute (less than few days) and the chronic (more than 4 weeks) phases of injury via cell adhesion molecules, chemokines and chemoattractants for infiltrating immune cells. In contrast, Detloff and colleagures reported TNFα and IL-1β increased in spinal tissue in the lumbar dorsal horn on POD 7 and returned at basal level on POD35 after thoracic contusion injury (Detloff et la., 2008).
Chemokines
Chemokines are a family of chemoattractive cytokines. Based on data from in situ hybridization and immunohistochemical studies, it is known that SCI acutely results in increased chemokines, such as MIP-1 and MCP-1, within a few minutes to 1 hour (Bartholdi and Schwab, 1997; Lee et al., 2000; Pineau et al., 2010). The production of chemokines is not restricted to specific regions, i.e. near injured site of the spinal cord, rather, chemokines are induced in remote regions of the spinal cord and thalamus (Knerlich-Lukoschus et al, 2008; Zhao et al., 2007a). Among chemokines, fractalkine is an excellent candidate for induction and persistent microglial activation. Neurons and astrocytes release fractalkines (CX3CL1) whereas microglia express the ligand receptor, CX3CR1. The fractalkine induced microglial activation is mediated by Cathepsin S, a cysteine protease which is on microglia (Clark et al., 2009; Linda et al., 2005; Verge et al., 2004). In addition, activation of CXCR4 by SDF1-α (CXCL12) in astrocytes induces calcium dependent release of glutamate that triggers activation of microglia (Bezzi et al., 2001). Taken together, chemokines are important candidates for astrocytic/neuronal-microglial interaction. In astrocytic-neuronal communication, the chemokine MCP-1 is an excellent example. Astrocytes primarily release MCP-1 and neurons express the ligand receptor CCL2, which suggests an obvious astrocytic-neuronal interaction.
Immune Cells
Immune cells are involved in defensive mechanisms against foreign or infectious materials in the body system and are activated by SCI. The activation of immune cells, more specifically infiltrating immune cells, such as polymorphonuclear leukocytes (PMN, neutrophils), macrophages and T-cells, develop at least 2 hours after injury and last over 6 months (Beck et al., 2010; Esposito et al., 2010; Nguyen et al., 2011). Activated and infiltrating neutrophils release proinflammatory cytokines, chemokines and reactive oxygen species (ROS) that contribute to lipid peroxidation and breakdown of blood-brain barrier (BBB) following SCI (Bao et al., 2004; Taoka and Okajima, 2000), suggesting that oxidative stress produced as a consequence of SCI plays a role in the breakdown of the BBB followed by infiltration of neutrophils from blood near the injury site (Lin et al., 2007; Schnell et al., 1999). Activated/infiltrating neutrophils also influence eicosanoid metabolism via leukotriene B4 after SCI (inhibition of leukotrienes B4 and LTB4, which suppresses neutrophil infiltration after SCI, Saiwai et al., 2010). The mechanisms of neutrophil and cell communication are not clear, but it is possible that beta2-integrin of neutrophils bind to vascular adhesion molecule-1 (VCAM-1) and contribute to trafficking of cell-cell interactions and infiltration during inflammation after SCI (Mabon et al., 2000). In addition, activated macrophages also release proinflammatiory cytokines, ROS, prostaglandins (PGs) and cathepsin S, which are involved in microglial activation (Liuzzo et al., 1999). Another immune cell class, the T-cells, also infiltrate the injured spinal cord from circulating blood and release proinflammatory cytokines to stimulate neighboring glial cells. Recent literature reports that T cells are not the major component for acute (few days after SCI) inflammatory responses after SCI, rather that they are actively involved in the later phase of glial activation (Stirling and Yong, 2008). Taken together, proinflammatory cytokines, chemokines and immune cells interactively contribute to both acute and persistent glial activation in the spinal cord following SCI.
4. Glial activation by cell cycle proteins after SCI
SCI produces altered expression of cell cycle proteins in neurons and glial cells (Di Giovanni et al., 2003; Saito et al., 2000). Over-expression of cell cycle proteins directly affects apoptosis-mediated neuronal death and glial proliferation (Byrnes et al., 2007; Di Giovanni et al., 2005; Ji et al., 2008). Among cell cycle proteins, cyclin-dependent kinase (CDKs) inhibitors are critically involved in the control of the cell cycle, apoptosis and proliferation of neural cells in the central nervous system (Fischer et al., 2003). Recent data suggests that cell cycle proteins regulate the mechanism of glial activation/proliferation following SCI. For example, acute (1 hour after SCI) intraperitoneally administration of the cell cycle inhibitor olomoucine, a competitive CDK inhibitor at late G1 phase of the cell cycle (Glab et al., 1994), inhibited early microglial activation/proliferation (1 and 3 days after SCI) and maintained astrogliosis (1 week to 4 weeks after SCI) as well as release of pro-inflammatory cytokines, such as TNFα and IL-1β following T12 spinal transection injury (Tian et al., 2007a,b). In addition, SCI produces down regulation of p27kip1, a member of Cip/Kip family of CDK inhibitors, within 3 days after injury, which correlates well with astrocytic and microglial proliferation via upregulation of KPC1, a regulator of P27kip1 degradation during the G1 phase of cell division (Shen et al., 2008; Zhao et al., 2011). P27kip1 regulates cell cycle processes via inhibition of the cyclin/CDK2 complex and down regulation of P27kip1 leads to increased neuronal and glial proliferation following SCI.
Glial Activation on Central Neuropathic Pain Following SCI
The majority of people with SCI suffer from devastating chronic neuropathic pain syndromes that are so severe that depression, drug abuse and suicide often result (Cairnes et al., 1996; Harris et al., 1996; McKinley et al., 1999). SCI produces dramatic alterations in synaptic circuits relaying somatotopic sensory transmission in the dorsal horn. The neuroanatomical and neurochemical changes in synaptic circuits directly affect postsynaptic membrane excitability and activation of intracellular downstream cascades that result in enhanced evoked and spontaneous electrophsiological activity in dorsal horn neurons. The abnormally increased neuronal activity is called hyperexcitability of dorsal horn neurons or central sensitization of neurons in the dorsal horn, a representative spinal mechanism of central neuropathic pain (CNP) following SCI (Gwak et al., 2008, Gwak and Hulsebosch, 2010a).
Glial cells play important roles in the maintenance of neuroarchitectural structure and physiological homeostasis in the spinal cord (Mills et al., 2004), brain stem (Koshinaga and Whittemore, 1995), thalamus (Hains and Waxman, 2007) and cortex (Rathinam et al., 2006). Astrocytes communicate with neighboring neurons via synapses and gap junctions (Theriault et al., 1997). The radial branches of astrocytes synapse with single and/or several neurons or microglia; contributing to multiple astrocytic-neuronal and astocytic-microglial communication possibilities; whereas, microglia are freely motile and primarily play important roles in the endogenous immune responses (Bezzi et al., 2001; Mukaino et al., 2010). The neuroarchitectural arrangement of the glial structures suggest that if one cell is activated, it is able to influence near by neuronal cells, by either inhibitory or excitatory mechanisms. For over a decade, studies show that SCI produces glial activation in the dorsal horn and contributes to the primary and secondary damage followed by maladaptive sensory and motor abnormalities in the dorsal horn (Lytle and Wrathall, 2007; Smith and Strunz, 2005). While the role of activated glial cells in peripheral neuropathic pain states are documented after peripheral nerve injury, there are few studies that focus on glial contribution with respect to central neuropathic pain after spinal cord injury. In a very early report, Garrison et al. reported that increased GFAP, i.e., somatic hypertrophy, in the dorsal horn was well correlated with hyperalgesic behavior following sciatic nerve injury (Garrison et al., 1991). Peripheral glial cells, such as Schwann cells, produce TNFα that contributes to hyperalgesia following compression of peripheral nerve (Wagner and Myers, 1996). The direct evidence that activated astrocytes and microglia are important contributors to neuropathic pain are the following: 1) Colburn et al. report that several peripheral nerve injury models demonstrate glial activation and mechanical allodyia and thermal hyperalgesia behavior (Colburn et al., 1997, 1999). 2) Earlier studies, detailed descriptions of acute (less than few days) and persistent (over a month) activated glial roles on pathophysiological pain, suggesting that glial cell activation was essential for the development and maintenance of neuropathic pain in several pathological conditions, such as peripheral nerve injury, inflammation and bone cancer pain (Hua et al., 2005; Zhang et al., 2005).
With respect to SCI studies, both spinal contusion and hemisection injury models demonstrate that activated glial cells in the dorsal horn are important factors for central neuropathic pain induced by SCI (see below).
1. Contusion Injury
In earlier reports, both spinal hemisection and spinal contusion injury resulted in increased NGF levels on spinal cord and blood serum,, presumably from activated microglia and macrophages (both resident and circulating), that contributes to sprouting of fine primary afferent fibers and abnormal pain processing in the dorsal horn (Christensen and Hulsebosch, 1997; Bennett et al., 1999; Krenz and Weaver, 2000). Elevated NGF also facilitates expression of IL-6 in macrophages, which are involved in the chronic phase of inflammation. In addition, activated microglia release BDNF which involves a shift in anion equilibrium potential (Eanion) via activation of tyrosine kinase receptor (TrkB). The Eanion shift generates depolarizing rather than hyperpolarizing membrane potentials, which contributes to mechanical allodynia (Coull et al., 2005). Hains and his colleagues suggested a more detailed spatial mechanism for glial contribution to central neuropathic pain following SCI. After T9 contusion injury, activated microglia play important roles in both behavioral sensitivity in hindpaws and lumbar neuronal hyperexcitability via activation of p38 MAPK and ERK1/2 activation (phosphorylation), suggesting that activated microglia play important roles in below-level neuropathic pain. Activation of the MAPK family triggers release of prostaglandin E2 (PGE2) and facilitates cyclooxygenase-2 (COX-2) expression in activated microglia in both the dorsal horn and the thalamic VPL regions, which results in neuronal hyperexcitability (Hains and Waxman, 2006; Zhao et al., 2007a). In addition, inhibition of cystein-cysteine chemokine ligand 21 (CCL21) and minocycline treatment produced inhibition of microglial activation and attenuation of neuronal hyperexcitability in the lumbar spinal dorsal horn neurons and thalamic VPL neurons, respectively (Tan et al., 2009; Zhao et al., 2007b). Other groups also reported that spinal contusion injury produced microglial activation with increased production of TNFα and IL-1β in both lumbar dorsal horn and thalamic VPL regions (Detloff et al., 2008). In addition, we report that T10 contusion injury produces mechanical and thermal pain behaviors as well as hyperexcitability in dorsal horn neurons in cervical spinal regions, accompanied by astrocytic and microglial activation (Carlton et al., 2009). It is well documented that SCI causes high concentrations of extracellular glutamate in the extracellular space and synaptic clefts (McAdoo et al., 1999). Glial glutamate transporters, such as GLAST (EAAT1) and GLT-1 (EAAT2) control extracellular glutamate concentrations by moving glutamate into the intracellular compartments of astrocytes via activation of nuclear factor-kappaB (Zelenaia et al., 2000). Previously, we reported that T10 contusion injury acutely (within 1 hour) increases GLT-1 (EAAT2) and GLAST (EAAT1) expression in the dorsal horn (Vera-Portocarrero et al., 2002) whereas, other studies report decreased GLT-1 expression at 1 day to 2 weeks after T10 compression injury in rats (Olsen et al., 2010), T9 contusion injury in mice (Lepore et al., 2011) and spinal cord ischemic injury in rats (Chen et al., 2010), suggesting that neurons and activated microglia contribute to the changes in glial transporter expression following SCI. For example, minocycline treatment prevents downregulation of GLT-1 and GLAST as well as inhibits astrocytic activation (Nie et al., 2010). In addition, inhibition of IL-1 and ROS prevents downregulation of GLT-1 and GLAST expression by Ca2+-dependent phosphoinositide 3-kinase-P2Y7R coupling in astocytes following T9/10 contusion injury (Liu et al., 2010; Prow and Irani, 2008; Zagami et al., 2005). Dysregulation of glial glutamate transporters causes increased concentrations of extracellular glutamate and produces glutamate-mediated neuronal hyperexcitability and/or excitotoxic cell death via ionotropic and metabotropic glutamate receptors following SCI.
2. Hemisection Injury
Unilateral T13 hemisection produces bilateral microglial activation and increased TNFα expression (3 days to over 2 weeks) in the lumbar dorsal horn which correlates well with mechanical allodynia in the hindpaws (Pan et al., 2003; Peng et al., 2006). Previously, we demonstrated that T13 spinal hemisection produced bilateral activation of astrocytes and microglia, neuronal hyperexcitability and downregulation of GAD65 in the lumbar dorsal horn 4 weeks POD. In addition, T13 hemisection produces activation of p38 MAPK in both neurons and microglia in the lumbar dorsal horn. Treatment with propentofylline, a phosphdiesterase inhibitor and glial modulator, prevented both microglial and astrocytic activation, attenuated neuronal hyperexcitability and attenuated increased mechanical and thermal sensitivities to stimuli (Gwak et al., 2008, 2009; Gwak and Hulsebosch, 2009). In addition, immediate treatment with TNFα blocker or minocycline treatment prevented development of T13 hemisection-induced microglial activation and mechanical allodynia via inhibition of microglial p38 MAPK activation (Marchand et al., 2009; Peng et al., 2006). Recently, it is reported that activation of phosphorylated NR1 contributes to astocytic activation and neuropathic pain in the lumbar dorsal horn following T13 hemisection injury (Roh et al., 2010).
In vivo and in vitro SCI studies suggest that activated astrocytes and microglia are the key factors for the development and maintenance of central neuropathic pain following SCI (Gwak et al., 2008; Hains and Waxman, 2006; Roh et al., 2010). In vivo behavioral studies demonstrate that intrathecal treatment with glial cell inhibitor agents, such as the microglial inhibitors minocycline and propentophylline, or a potent gap junction blocker carbenoxolone all attenuate SCI-induced mechanical allodynic behaviors following SCI. In vivo electrophysiological studies, further demonstrate that treatment with minocycline or propentophylline onto the spinal surface attenuated SCI-induced evoked hyperexcitability of spinal dorsal horn neurons in response to mechanical stimuli including non-noxious and noxious intensities applied to isolated peripheral receptive fields (Gwak and Hulsebosch, 2009; Hains and Waxman, 2006). In vitro immunohistochemical studies demonstrate that the astrocytic and microglial hypertrophy produced by SCI were restored to their quiescent state morphology in the spinal dorsal horn after minocycline, propentophylline, or carbenoxolone treatments, respectively. Pharmacologically, minocycline and propentophylline both inhibit interleukins-1, -6 and -8 as well as TNFα, which then result in inhibition of posttranscriptional processes that result in abnormal expression of target proteins, such as upregulation of glutamate receptors and sodium channels in neurons and microglia (Hains et al., 2003; Mills et al., 2002). Activated glia cells release proinflammatory cytokines, reactive oxygen species (ROS), glutamate and ATP followed by initiation of glutamate receptor activation at postsynaptic neurons leading to massive influx of Ca2+ ions into the intracellular space triggering multiple posttranslational processes (Bastos et al., 2007; Padi and Kulkarni, 2008; Pineau and Lacroix, 2007; Zhang et al., 2008). For example, spinal contusion injury at T10 produces activation of transcriptional factors, such as CREB and NF-κB, in the spinal dorsal horn followed by increases of iNOS and COX-2 production that contribute to neuronal hyperexcitability and central neuropathic pain (Crown et al., 2006; Rafati et al., 2008). These data collectively indicate that activated glial cells modulate neurotransmitters and/or proinflammatory cytokines, induce glutamate receptor/ion channel coupling that result in central neuropathic pain behavior as a result of dorsal horn neuronal hyperexcitability following SCI.
In summary, SCI produces astrocytic and microglial activation that extends the entire neuraxis of the spinal dorsal horn and contributes to both the development and the maintenance of above-, at-, and below-level neuropathic pain following SCI. Because the glial functions are considerably altered, become dysfunctional and persist after SCI, the result is neuropathic pain that severely alters the quality of life. Thus, SCI and other neurodegenerative disease conditions can be characterized as a result of dysfunctional glial populations or “gliopathy” (Hulsebosch, 2008; Gwak and Hulsebosch, 2010b). Consequently, therapy that lessens the gliopathic response should attenuate neuropathic pain symptoms.
Conclusion
A methodological tool to detect astrocytic and microglial cell types is the use of cell specific antibody-antigens reaction, such as glial fibrillary acid protein (for astrocytes) and OX-42 (for microglia). The morphological changes, such as somatic hypertrophy, proliferation, thickened branches, are common morphological features and correlate well with behavioral, electrophysiological and molecular changes of the altered somatosensory system, and result in chronic neuropathic pain following SCI. The collective data in SCI studies suggest that glial activation influences the neuronal-glial and glial-glial communication and alter the “gain” in response to sensory stimuli at the level of synaptic circuits following SCI. There is some controversy, but our work demonstrates that SCI produces glial activation at both acute (2 hour to a few days) and persistent (over 3 months) times in the spinal dorsal horn along the entire spinal axis (however, see Lepore et al., 2011). Our work demonstrates causality in that inhibition of glial activation (both microglia and astrocytic) after SCI attenuates the abnormal hyperexcitability of dorsal horn neurons to non-noxious and noxious stimuli, which is the substrate for persistent neuropathic pain (Gwak et al., 2009; Gwak and Hulsebosch, 2009). Our work in the context of work by others in SCI models demonstrate that dysfunctional glia, a condition called “gliopathy”, are key contributors in the underlying cellular mechanisms contributing to neuropathic pain (Hulsebosch, 2008; Gwak and Hulsebosch, 2010b).
Highlights.
* SCI produces consistent central neuropathic pain and glial activation in the dorsal horn
* Activated glial cells strongly interact with neuronal cells and directly cause maladaptive synaptic circuits
* Inhibition of glail activation is a strategy to alleviate CNP
Acknowledgements
This work was supported by Liddell Grant, West and Dunn Foundations and NIH NS11255 and NS39161 and Army PRO43199, Mission Connect, a project of the TIRR Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Author's have no conflict of interest
References
- Andrade MS, Hanania FR, Daci K, Leme RJ, Chadi G. Contuse lesion of the rat spinal cord of moderate intensity leads to a higher time-dependent secondary neurodegeneration than severe one. An open-window for experimental neuroprotective interventions. Tissue Cell. 2008;40:143–156. doi: 10.1016/j.tice.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010;229:26–50. doi: 10.1016/j.jneuroim.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Baldwin ST, Broderick R, Blades DA, Scheff ST. Alteration in temporal/spatial distribution of GFAP-and Vimentin-positive astrocytes after spinal cord contusion with the New York University Spinal Cord Injury Device. J. Neurotrauma. 1998;15:1015–1026. doi: 10.1089/neu.1998.15.1015. [DOI] [PubMed] [Google Scholar]
- Ballestas ME, Benveniste EN. Interleukin 1-beta- and tumor necrosis factor-alpha-mediates regulation of ICAM-1 gene expression in astrocytes requires protein kinase C activity. Glia. 1995;14:267–268. doi: 10.1002/glia.440140404. [DOI] [PubMed] [Google Scholar]
- Baloui H, Stettler O, Weiss S, Nothias F, von Boxberg Y. Upregulation in rat spinal cord microglia of the non integrin laminin receptor 37 kDa-LRP following activation by a traumatic lesion or peripheral injury. J. Neurotrauma. 2009;26:195–207. doi: 10.1089/neu.2008.0677. [DOI] [PubMed] [Google Scholar]
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J. Neurochem. 2004;88:1335–1344. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Ghirri A, Zonta M, Betelli C, Vitale G, Ruggieri V, Sandrini M, Carmignoto G. Glutamate-mediated astrocyte-to-neuron signalling in the rat dorsal horn. J. Physiol. 2010;588:831–846. doi: 10.1113/jphysiol.2009.180570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos LF, Merlo LA, Rocha LT, Coelho MM. Characterization of the antinociceptive and anti-inflammatory activities of doxycycline and minocycline in different experimental models. Eur. J. Pharmacol. 2007;576:171–179. doi: 10.1016/j.ejphar.2007.07.049. [DOI] [PubMed] [Google Scholar]
- Bartholdi D, Schwab ME. Expression of pro-inflammatory cytokines and chemokine mRNA upon experimental spinal cord injury in mouse: an in situ hybridization study. Eur. J. Neurosci. 9:1422–1438. doi: 10.1111/j.1460-9568.1997.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguygen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AS, Schousboe A, Reichelt W, Norenberg MD. Ionic mechanisms in glutamate-induced astrocyte swelling: role of K+ influx. J. Neurosci. Res. 1998;52:307–321. doi: 10.1002/(SICI)1097-4547(19980501)52:3<307::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Taglialatela G, Perez-Polo R, Hulsebosch CE. NGF levels decrease in the spinal cord and dorsal root ganglion after spinal hemisection. Neuroreport. 1999;10:889–893. doi: 10.1097/00001756-199903170-00040. [DOI] [PubMed] [Google Scholar]
- Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000;859:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 2011;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. 2007. [DOI] [PubMed]
- Cairns DM, Adkins RH, Scott MD. Pain and depression in acute traumatic spinal cord injury: origins of chronic problematic pain? Arch. Phys. Med. Rehabil. 1996;77:329–335. doi: 10.1016/s0003-9993(96)90079-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peipheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadi G, Andrade MS, Leme RJ, Gomide VC. Experimental models of partial lesion of rat spinal cord to investigate neurodegeneration, glial activation, and behavior impairments. Int. J. Neurosci. 2001;111:137–165. doi: 10.3109/00207450108994227. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Peterson PK. Glia, cytokines, and neurotoxicity. Crit. Rev. Neurobiol. 1995;9:189–205. [PubMed] [Google Scholar]
- Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur. J. Neurosci. 2005;22:1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- Chen WF, Sung CS, Jean YH, Su TM, Wang HC, Ho JT, Huang SY, Lin CS, Wen ZH. Suppressive effects of intrathecal granulocyte colony-stimulating factor on excessive release of excitatory amino acids in the spinal cerebrospinal fluid of rats with cord ischemia: role of glutamate transporters. Neuroscience. 2010;165:1217–1232. doi: 10.1016/j.neuroscience.2009.11.033. [DOI] [PubMed] [Google Scholar]
- Choi DC, Lee JY, Moon YJ, Kim SW, Oh TH, Yune TY. Acupuncture-mediated inhibition of inflammation facilitates significant functional recovery after spinal cord injury. Neurobiol. Dis. 2010;39:272–282. doi: 10.1016/j.nbd.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Hulsebosch CE. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 1997;147:463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Malcangio M. The liberation of fractalkine in the dorsal horn requires microglia cathepsin S. J. Neurosci. 2009;29:3675–6954. doi: 10.1523/JNEUROSCI.0828-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Rickman AJ, DeLeo JA. The effect of site and type of nerve injury on spinal glial activation and neuropathic pain behavior. Exp. Neurol. 1999;157:289–304. doi: 10.1006/exnr.1999.7065. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J. Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP Kinase is involved in central neuropathic pain following spinal cord injury. Exp. Neurol. 2008;213:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2008;212:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni S, Movsesyan V, Ahmed F, Cernak I, Schinelli S, Stoica B, Faden AI. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. 2005;102:8333–8338. doi: 10.1073/pnas.0500989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann. Neurol. 2003;53:454–468. doi: 10.1002/ana.10472. [DOI] [PubMed] [Google Scholar]
- Dihné M, Block F, Korr H, Töpper R. Time course of glial proliferation and glial apoptosis following excitotoxic CNS injury. Brain Res. 2001;902:178–189. doi: 10.1016/s0006-8993(01)02378-2. [DOI] [PubMed] [Google Scholar]
- Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Mazzon E, Paterniti I, Impellizzeri D, Bramanti P, Cuzzocrea S. Olprinone attenuates the acute inflammatory response and apoptosis after spinal cord trauma in mice. PLoS One. 2010;5:e12170. doi: 10.1371/journal.pone.0012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulenburg V, Gomeza J. Neurotransmitter transporters expressed in glial cells as regulators of synapse function. Brain Res. Rev. 2010;63:103–112. doi: 10.1016/j.brainresrev.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Fam SR, Gallagher CJ, Salter MW. P2Y1 purinoceptor-mediated Ca2+ signaling and Ca2+ wave propagation in dorsal spinal cord astrocytes. J. Neurosci. 2000;20:2800–2808. doi: 10.1523/JNEUROSCI.20-08-02800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer PM, Endicott J, Meijer L. Cyclin-dependent kinase inhibitors. Prog. Cell Cycle Res. 2003;5:235–248. [PubMed] [Google Scholar]
- Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki M, Zhang Z, Guth L, Steward O. Genetic influences on cellular reactions to spinal cord injury: activation of macrophage/microglia and astrocytes is delayed in mice carrying a mutation (WidS) that cause delayed Wallerian degeneration. J. Comp. Neurol. 1996;371:469–484. doi: 10.1002/(SICI)1096-9861(19960729)371:3<469::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010;58:1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- Gitter BD, Regoli D, Howbert JJ, Glasebrook AL, Waters DC. Interleukin-6 secretion from human astrocytoma cells induced by substance P. J. Neuroimmunol. 1994;51:101–108. doi: 10.1016/0165-5728(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Glab N, Labidi B, Qin LX, Trehin C, Bergounioux C, Meijer L. Olomoucine, an inhibitor of the cdc2/cdk2 kinases activity, blocks plant cells at the G1 to S and G2 to M cell cycle transitions. FEBS Lett. 1994;353:207–211. doi: 10.1016/0014-5793(94)01035-8. [DOI] [PubMed] [Google Scholar]
- Gomes-Leal W, Corkill DJ, Freire MA, Picanço-Diniz CW, Perry VH. Astrocytosis, microglia activation, oligodendrocyte degeneration, and pyknosis following acute spinal cord injury. Exp. Neurol. 2004;190:456–467. doi: 10.1016/j.expneurol.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J. Neurosci. Res. 1989;22:103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2010a;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. “Gliopathy” Maintains Persistent Hyperexcitability of Spinal Dorsal Horn Neurons after Spinal Cord Injury: Substrate of Central Neuropathic Pain. In: Costa A, Villalba E, editors. Horizons in Neuroscience Research. I. Nova Science Publishers; 2010b. pp. 195–224. 2010. [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote Astrocytic and Microglial Activation Modulate Neuronal Hyperexcitability and Below-Level Neuropathic Pain after Spinal Injury in Rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Unabia GC, Hulsebosch CE. Activation of p-38alpha MAPK contributes to neuronal hyperexcitability in caudal regions remote from spinal cord injury. Exp. Neurol. 2009;220:154–161. doi: 10.1016/j.expneurol.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;138:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber M, Zhou L, Murai KK. Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J. Neurosci. 2006;26:8881–8891. doi: 10.1523/JNEUROSCI.1302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Klein JP, Saab CY, Craner MJ, Black JA, Waxman SG. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003;23:8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EC, Barraclough BM, Grundy DJ, Bamford ES, Inskip HM. Attempted suicide and completed suicide in traumatic spinal cord injury. Case reports. Spinal Cord. 1996;34:752–753. doi: 10.1038/sc.1996.138. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp. Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacque CM, Vinner C, Kujas M, Raoul M, Racadot J, Baumann NA. Determination of glial fibrillary acidic protein (GFAP) in human brain tumors. J. Neurol. Sci. 1978;35:147–155. doi: 10.1016/0022-510x(78)90107-7. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Liu M, Moriyama M, Ramakrishnan R, Forbush B, 3rd, Reddy PV, Norenberg MD. Na-K-Cl Cotransporter-1 in the mechanism of ammonia-induced astrocyte swelling. J. Biol. Chem. 2008;283:33874–33882. doi: 10.1074/jbc.M804016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF. The neural-glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Jendelová P, Syková E. Role of glia in K+ and Ph homeostasis in the neonatal rat spinal cord. Glia. 1991;4:56–63. doi: 10.1002/glia.440040107. [DOI] [PubMed] [Google Scholar]
- Ji Y, Xiao F, Sun L, Qin J, Shi S, Yang J, Liu Y, Zhou D, Zhao J, Shen A. Increased expression of CDK11P58 and cyclin D3 following spinal cord injury in rats. Mol. Cell. Biochem. 2008;309:49–60. doi: 10.1007/s11010-007-9642-z. [DOI] [PubMed] [Google Scholar]
- Jin Y, Neuhuber B, Singh A, Bouyer J, Lepore A, Bonner J, Himes T, Campanelli JT, Fischer I. Transplantation of human glial restricted progenitors and derived astrocytes into a contusion model of spinal cord injury. J. Neurotrauma. 2011;28:579–594. doi: 10.1089/neu.2010.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, Basso DM, Sodhi A, Pan PZ, Hart RP, MacCallum RC, Lee S, Whitacre CC, Popovich PG. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J. Neurosci. 2002;22:2690–2700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K, Min DS, Sim KB, Ahn M, Kim H, Cheong J, Shin T. Upregulation of phospholipase D1 in the spinal cord of rats with clip compression injury. Neurosci. Lett. 2003;336:126–130. doi: 10.1016/s0304-3940(02)01155-2. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, Juraschek M, Blömer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J. Neurotrauma. 2008;25:427–448. doi: 10.1089/neu.2007.0431. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Spatiotemporal CCR1, CCL3(MIP-1α), CXCR4, CXCL12(SDF-1α) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J. Neurosurg. Spine. 2011;14:583–597. doi: 10.3171/2010.12.SPINE10480. [DOI] [PubMed] [Google Scholar]
- Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Chemokine expression in the white matter spinal cord precursor niche after force-defined spinal cord contusion injuries in adult rats. Glia. 2010;58:916–931. doi: 10.1002/glia.20974. [DOI] [PubMed] [Google Scholar]
- Korn T, Magnus T, Jung S. Interaction with antigen-specific T cells regulates expression of the lactate transporter MCT1 in primary rat astrocytes: specific link between immunity and homeostasis. Glia. 2005;49:73–73. doi: 10.1002/glia.20101. [DOI] [PubMed] [Google Scholar]
- Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tacts undergoing anterograde and retrograde degeneration following spinal cord lesion. J. Neurotrauma. 1995;12:209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- Krenz NR, Weaver LC. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J. Neurochem. 2000;74:730–739. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- Kyrkanides S, Olschowka JA, Williams JP, Hansen JT, O'Banion MK. TNF alpha and IL-1beta mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. J. Neuroimmunol. 1999;95:95–106. doi: 10.1016/s0165-5728(98)00270-7. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Lee YL, Shih K, Bao P, Ghimikar RS, Eng JF. Cytokine chemokine expression in contused rat spinal cord. Neurochem. Int. 2000;36:417–425. doi: 10.1016/s0197-0186(99)00133-3. [DOI] [PubMed] [Google Scholar]
- Lepore AC, O'Donnell J, Bonner JF, Paul C, Miller ME, Rauck B, Kushner RA, Rothstein JD, Fischer I, Maragakis NJ. Spatial and temporal changes in promoter activity of the astrocyte glutamate transporter GLT1 following traumatic spinal cord injury. J. Neurosci. Res. 2011;89:1001–1017. doi: 10.1002/jnr.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Hogan EL, Banik NL. Role of calapin in spinal cord injury: increased mcalpain immunoreactivity in spinal cord after compression injury in the rat. Neurochem. Int. 1995;27:425–432. doi: 10.1016/0197-0186(95)00024-3. [DOI] [PubMed] [Google Scholar]
- Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc. Natl. Acad. Sci. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Vreman HJ, Wong RJ, Tjoa T, Yamauchi T, Noble-Haeusslein LJ. Heme oxygenase-1 stabilizes the blood-spinal cord barrier and limits oxidative stress and white matter damage in the acutely injured murine spinal cord. J. Cereb. Blood Flow Metab. 2007;27:1010–1021. doi: 10.1038/sj.jcbfm.9600412. [DOI] [PubMed] [Google Scholar]
- Linda JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J. Pain. 2005;6:434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Liu YP, Yang CS, Chen MC, Sun SH, Tzeng SF. Ca(2+)-dependent reduction of glutamate aspartate transporter GLAST expression in astrocytes by P2X(7) receptor-mediated phosphoinositide 3-kinase signaling. J. Neurochem. 2010;113:213–227. doi: 10.1111/j.1471-4159.2010.06589.x. [DOI] [PubMed] [Google Scholar]
- Liuzzo JP, Petanceska SS, Devi LA. Neurotropic factors regulate cathepsin S in microphage and microglia: a role in the degradation of myelin basic protein and amyloid beta peptide. Mol. Med. 1999;5:334–343. [PMC free article] [PubMed] [Google Scholar]
- Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur. J. Neurosci. 2007;25:1711–1724. doi: 10.1111/j.1460-9568.2007.05390.x. [DOI] [PubMed] [Google Scholar]
- Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphD: a potential new anti-inflammatory treatment. Exp. Neurol. 2000;66:52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur. J. Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Matsumoto M, Yamashita A, Ohtaka K, Ishida K, Morimoto Y, Sakabe T. Anesth. Analg. 2003;96:1777–1784. doi: 10.1213/01.ANE.0000064204.67561.73. [DOI] [PubMed] [Google Scholar]
- McAdoo DJ, Xu GY, Robak G, Hughes M. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp. Neurol. 1999;159:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;15:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- McKinley WO, Kolakowsky SA, Kreutzer JS. Substance abuse, violence, and outcome after traumatic spinal cord injury. Am. J. Phys. Med. Rehabil. 1999;78:306–312. doi: 10.1097/00002060-199907000-00002. [DOI] [PubMed] [Google Scholar]
- Mills LR, Velumian AA, Agrawal SK, Theriault E, Fehlings MG. Confocal imaging of changes in glial calcium dynamics and homeostasis after mechanical injury in rat spinal cord white matter. Neuroimage. 2004;21:1069–1082. doi: 10.1016/j.neuroimage.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Mills CD, Johnson KM, Hulsebosch CE. Role of group II and group III metabotropic glutamate receptors in spinal cord injury. Exp. Neurol. 2002;173:153–167. doi: 10.1006/exnr.2001.7828. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Jayakumar AR, Tong XY, Norenberg MD. Role of mitogen-activated protein kinases in the mechanism of oxidant-induced cell swelling in cultured astrocytes. J. Neurosci. Res. 2010;88:2450–2458. doi: 10.1002/jnr.22400. [DOI] [PubMed] [Google Scholar]
- Mukaino M, Nakamura M, Yamada O, Okada S, Morikawa S, Renault-Mihara F, Iwanami A, Ikegami T, Ohsugi Y, Tsuji O, Katoh H, Matsuzaki Y, Toyama Y, Liu M, Okano H. Exp. Neurol. 2010;224:403–414. doi: 10.1016/j.expneurol.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kaneko S. Spinal astrocytes as therapeutic targets for pathological pain. J Pharmacol Sci. 2010;114:347–353. doi: 10.1254/jphs.10r04cp. [DOI] [PubMed] [Google Scholar]
- Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science. 1994;263:1768–1771. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]
- Nesic O, Lee J, Johnson KM, Y,e. Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 2005;95:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- Nguyen HX, Beck KD, Anderson AJ. Quantitative assessement of immune cells in the injured spinal cord tissue by flow cytometry: a novel use for a cell purification method. J. Vis. Exp. 2011;9:2698. doi: 10.3791/2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Zhang H, Weng HR. Minocycline prevents impaired glial glutamate uptake in the spinal sensory synapses of neuropathic rats. Neuroscience. 2010;170:901–912. doi: 10.1016/j.neuroscience.2010.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Panatier A, Piet R, Mothet JP, Poulain DA, Theodosis DT. Neuron-glia interactions in the rat supraoptic nucleus. Prog. Brain. Res. 2008;170:109–117. doi: 10.1016/S0079-6123(08)00410-X. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Campbell SC, McFerrin MB, Floyd CL, Sontheimer H. Spinal cord injury causes a wide-spread, persistent loss of Kir4.1 and glutamate transporter 1: benefit of 17 beta-oestradiol treatment. Brain. 2010;133:1013–1025. doi: 10.1093/brain/awq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur. J. Pharmacol. 2008;601:79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium. 2010;48:225–231. doi: 10.1016/j.ceca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Pan W, Zhang L, Liao J, Csernus B, Kastin AJ. Selective increase in TNF alpha permeation across the blood-spinal cord barrier after SCI. J. Neuroimmunol. 2003;134:111–117. doi: 10.1016/s0165-5728(02)00426-5. [DOI] [PubMed] [Google Scholar]
- Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann. Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Pineau I, Sun L, Bastien D, Lacroix S. Astrocytes initiate inflammation in the injured mouse spinal cord by promoting the entry of neutrophils and inflammatory monocytes in a IL-1 receptor/MyD88-dependent fashion. Brain Behav. Immun. 2010;24:540–553. doi: 10.1016/j.bbi.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Pirttimaki TM, Hall SD, Parri HR. Sustained neuronal activity generated by glial plasticity. J. Neurosci. 2011;25:7637–7647. doi: 10.1523/JNEUROSCI.5783-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory responses after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J. Neurochem. 2008;105:1276–1286. doi: 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafati DS, Geissler K, Johnson K, Unabia G, Hulsebosch C, Nesic O, Perez-Polo JR. Nuclear factor-kappaB decoy amelioration of spinal cord injury-induced inflammation and behavior outcomes. J. Neurosci. Res. 2008;86:566–580. doi: 10.1002/jnr.21508. [DOI] [PubMed] [Google Scholar]
- Rathinam ML, Watts LT, Stark AA, Mahimainathan L, Stewart J, Schenker S, Henderson GI. Astrocyte control of fetal cortical neurons glutathione homeostasis: up-regulation by ethanol. J. Neurochem. 2006;96:1289–1230. doi: 10.1111/j.1471-4159.2006.03674.x. [DOI] [PubMed] [Google Scholar]
- Reme RJ, Chadi G. Distant microglial and astroglial activation secondary to experimental spinal cord lesion. Arq. Neuropsiquiatr. 2001;59:483–492. doi: 10.1590/s0004-282x2001000400002. [DOI] [PubMed] [Google Scholar]
- Rice T, Larsen J, Rivest S, Yong VW. Characterization of the early neuroinflammation after spinal cord injury in mice. J. Neuropathol. Exp. Neurol. 2007;66:184–195. doi: 10.1097/01.jnen.0000248552.07338.7f. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR, Waxman SG. Pharmacological characterization of Na+ influx via voltage-gated Na+ channels in spinal cord astrocytes. J. Neurophysiol. 1997;78:3249–3258. doi: 10.1152/jn.1997.78.6.3249. [DOI] [PubMed] [Google Scholar]
- Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2010;224:123–132. doi: 10.1016/j.expneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Saito N, Yamamoto T, Watanabe T, Abe Y, Kumagai T. Implication of p58 proteinexpression in experimental spinal cord injury. J. Neurotrauma. 2000;17:173–182. doi: 10.1089/neu.2000.17.173. [DOI] [PubMed] [Google Scholar]
- Saiwai H, Ohkawa Y, Yamada H, Kumamaru H, Harada A, Okano H, Yokomizo T, Iwamoto Y, Okada S. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci. Lett. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- Schnell L, Fearn S, Schwab ME, Perry VH, Anthony DC. Cytokine-induced acute inflammation in the brain and spinal cord. J. Neuropathol. Exp. Neurol. 1999;58:245–254. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci. 2007;10:1361–168. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen A, Liu Y, Zhao J, Qin J, Shuxian S, Chen M, Gao S, Xiao F, Lu Q, Cheng C. Temporal-spatial expression of p27kip1 and its phosphorylation on serine-10 after acute spinal cord injury in adults rat: Implication for post-traumatic glial proliferation. Neurochem. Int. 2008;52:1266–1275. doi: 10.1016/j.neuint.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Shi F, Zhu H, Yang S, Liu Y, Feng Y, Shi J, Xu D, Wu W, You S, Ma Z, Zou J, Lu P, Xu XM. Glial response and myelin clearance in areas of wallerian degeneration after spinal cord hemisection in the monkey Macaca fascicularis. J. Neurotrauma. 2009;26:2083–2096. doi: 10.1089/neu.2008.0706. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron. 2007;54:59–72. doi: 10.1016/j.neuron.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Song G, Cechvala C, Resnick DK, Dempsey RJ, Rao VL. GeneChip analysis after acute spinal cord injury in rat. J. Neurochem. 2001;79:804–815. doi: 10.1046/j.1471-4159.2001.00626.x. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J. Neurosci. 1994;14:2464–2475. doi: 10.1523/JNEUROSCI.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Liu J, Plunet W, Steeves JD, Tetzlaff W. SB203580, a p38 mitogen-activated protein kinase inhibitor, fails to improve functional outcome following a moderate spinal cord injury in rat. Neuroscience. 2008;155:128–137. doi: 10.1016/j.neuroscience.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury released by flow cytometry. J. Neurosci. Res. 2008;86:1944–1958. doi: 10.1002/jnr.21659. [DOI] [PubMed] [Google Scholar]
- Svensson M, Eriksson NP, Aldskogius H. Evidence for activation of astrocytes via reactive microglial cells following hypoglossal nerve transection. J. Neurosci. Res. 1993;35:373–381. doi: 10.1002/jnr.490350404. [DOI] [PubMed] [Google Scholar]
- Syková E, Chvátal A. Extracellular ionic and volume changes: the role in glia-neuron interaction. J. Chem. Neuroanat. 1993;6:247–260. doi: 10.1016/0891-0618(93)90046-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tsuruhara H. Slow depolarizing potentials recorded from glial cells in the rat superficial dorsal horn. J. Physiol. 1987;388:597–610. doi: 10.1113/jphysiol.1987.sp016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Zhao P, Waxman SG, Hains BC. Early microglial inhibition preemptively mitigates chronic pain development after experimental spinal cord injury. J. Rehabil. Res. Dev. 2009;46:123–133. [PubMed] [Google Scholar]
- Taoka Y, Okajima K. Role of leukocytes in spinal cord injury in rats. J. Neurotrauma. 2000;17:219–229. doi: 10.1089/neu.2000.17.219. [DOI] [PubMed] [Google Scholar]
- Theriault E, Frankenstein UN, Hertzberg EI, Nagy JI. Connexin43 and astrocytic gap junsctions in the rat spinal cord after acute compression injury. J. Comp. Neurol. 1997;382:199–214. [PubMed] [Google Scholar]
- Tian DS, Dong Q, Pan DJ, He Y, Yu ZY, Xie MJ, Wang W. Attenuation of astrogliosis by suppressing microglial proliferation with the cell cycle inhibitor olomoucine in rat spinal cord injury model. Brain Res. 2007a;1154:206–214. doi: 10.1016/j.brainres.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Tian DS, Xie MJ, Yu ZY, Zhang Q, Wang YH, Chen B, Chen C, Wang W. Cell cycle inhibition attenuates microglia induced inflammatory responses and alleviates neuronal cell death after spinal cord injury in rats. Bran Res. 2007b;1135:177–185. doi: 10.1016/j.brainres.2006.11.085. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng SF, Kahn M, Liva S, De Vellis J. Tumor necrosis factor-alpha regulation of the ld gene family in astrocytes and microglia during CNS inflammatory injury. Glia. 1999;26:139–152. doi: 10.1002/(sici)1098-1136(199904)26:2<139::aid-glia5>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10:167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Mills CD, Ye Z, Fullwood SD, McAdoo DJ, Hulsebosch CE, Westlund KN. Rapid changes in expression of glutamate transporters after spinal cord injury. Brain Res. 2002;927:104–110. doi: 10.1016/s0006-8993(01)03329-7. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain condition. Eur. J. Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Verdú E, García-Alías G, Forés J, López-Vales R, Navarro X. Olfactory ensheathing cells transplanted in lesioned spinal cord prevent loss of spinal cord parenchyma and promote functional recovery. Glia. 2003;42:275–286. doi: 10.1002/glia.10217. [DOI] [PubMed] [Google Scholar]
- Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamoto T, Abe Y, Saito N, Kumagai T, Kayama H. Differential activation of microglia after experimental spinal cord injury. J. Neurotrauma. 1999;16:255–265. doi: 10.1089/neu.1999.16.255. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Hains BC. Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 2006;29:207–215. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Weisshaar CL, Dong L, Bowman AS, Perez FM, Guarino BB, Sweitzer SM, Winkelstein BA. Metabotropic glutamate receptor-5 and protein kinaseC-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J. Neurotrauma. 2010;27:2261–2271. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- William R, Yao H, Dhillon NK, Busch SJ. HIV-1 Tat co-operates with IFN-gamma and TNF-alpha to increase CXCL10 in human astrocytes. PLoS One. 2009;28:e5709. doi: 10.1371/journal.pone.0005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmott NJ, Wong K, Strong AJ. A functional role for the nitric oxide-G-kinase signaling pathways in mediating intercellular Ca2+ waves in glia. J. Neurosci. 2000;20:1767–1779. doi: 10.1523/JNEUROSCI.20-05-01767.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Miyamoto O, Shibuya S, Mori S, Norimatsu H, Janjua NA, Itano T. Co-expression of radial glial marker in macrophage/microglia in rat spinal cord contusion injury model. Brain Res. 2005;1051:183–188. doi: 10.1016/j.brainres.2005.05.054. [DOI] [PubMed] [Google Scholar]
- Xu M, Ng YK, Leong SK. Induction of microglial reaction and expression of nitric oxide synthase I in the nucleus dorsalis and red nucleus following lower thoracic spinal cord hemisection. Brain Res. 1998;808:23–30. doi: 10.1016/s0006-8993(98)00787-2. [DOI] [PubMed] [Google Scholar]
- Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. 2004;29:966–971. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, Vidensky S, Brennan J, Poulsen D, Won Park J., Li Jeon. N., Robinson MB, Rothstein JD. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagami CJ, O'Shea RD, Lau CL, Cheema SS, Beart PM. Regulation of glutamate transporters in astrocytes: evidence for a relationship between transporter expression and astrocytic phenotype. Neurotox. Res. 2005;7:143–149. doi: 10.1007/BF03033783. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol. Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J. Neurosci. 2007a;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Modulation of thalamic nocieptive processing after spinal cord injury through remote activation of thalamic microglia by cysteine chemokine ligand21. J. Neurosci. 2007b;27:8893–8902. doi: 10.1523/JNEUROSCI.2209-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhang S, Wu X, Huan W, Liu Z, Wei H, Shen A, Teng H. KPC1 expression and essential role after acute spinal cord injury in adult rat. Neurochem. Res. 2011;36:549–558. doi: 10.1007/s11064-010-0377-y. [DOI] [PubMed] [Google Scholar]
- Zheng H, Zhu W, Zhao H, Wang X, Wang W, Li Z. Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: crucial role of interleukin-1beta. Neuroimmunomodulation. 2010;17:31–38. doi: 10.1159/000243083. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Zündorf G, Kahlert S, Reiser G. Gap-junction blocker carbenoxolone differentially enhances NMDA-induced cell death in hippocampal neurons and astrocytes in co-culture. J. Neurochem. 2007;102:508–521. doi: 10.1111/j.1471-4159.2007.04509.x. [DOI] [PubMed] [Google Scholar]