Scheme 3.
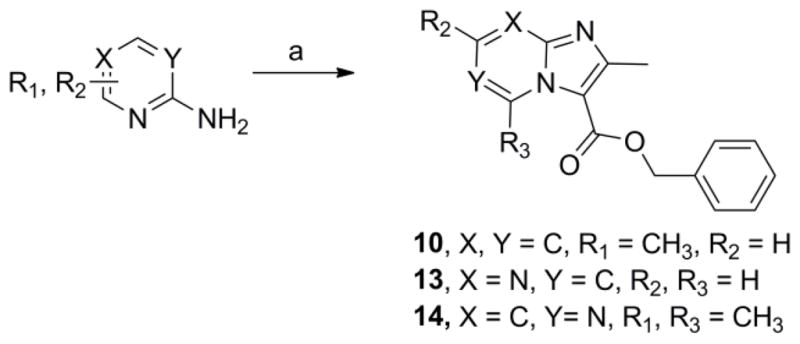
One step syntheses of benzyl 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxylate (10), benzyl 2-methylimidazo[1,2-a]pyrimidine-3-carboxylate (13) and benzyl 2,5,7-trimethylimidazo[1,2-c]pyrimidine-3-carboxylate (14). Reagents: (a) Benzyl 2-bromo-3-oxobutanoate, NaHCO3, 1,2-dimethoxyethane, 110°C, 24–36 h.
