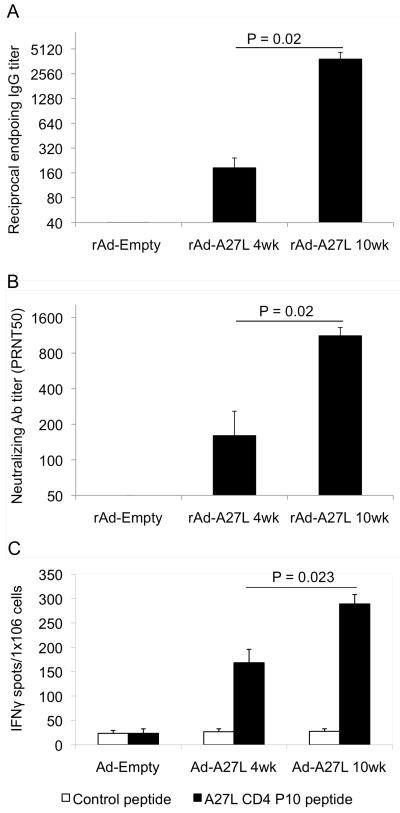
Fig. 3. Immunization with rAd-A27L generates potent cellular and antibody responses.
6 – 8 week old Balb/c mice were immunized i.m. with 1×109 pfu rAd-A27L or rAd-empty. Antibody and cellular immune responses were evaluated at 4 weeks and 10 weeks post vaccination. Pre-challenge sera were collected at one day prior to challenge. Serum IgG antibody titers (A) were evaluated against recombinant A27L protein by ELISA and neutralizing antibody titers (B) by PRNT50 assay. Endpoint antibody titers for the IgG ELISA were defined as the greatest serum dilutions having more than double the O.D. of pre-immune sera. Endpoint titers for the PRNT50 assay were defined as the greatest serum dilutions that caused a 50% reduction in numbers of plaques compared to the virus control wells. Spleen cells were harvested from immunized mice and used for analysis of cellular responses against the A27L CD4 P10 peptide by IFNγ-ELISPOT assay (C). Data are presented as numbers of IFNγ-positive spot forming cells (SFC) per million cells.