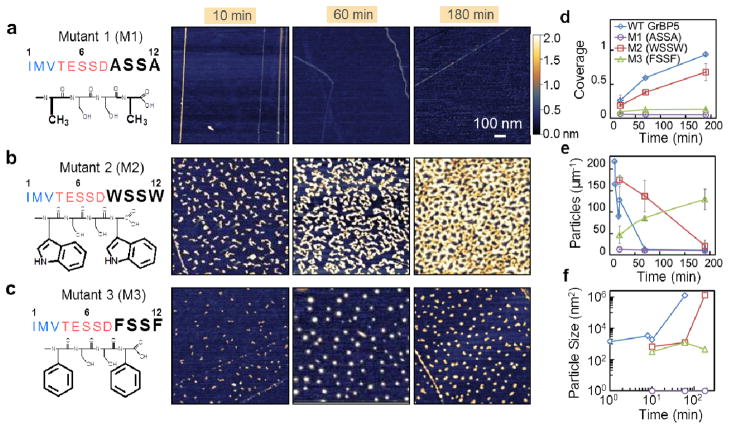
Figure 3.
Time-lapse behavior of the peptides with Domain-III mutations. (a) In Mutant 1 the aromatic residues, Tyrosine (Y), of GrBP5 are eliminated and replaced by Alanine (A) resulting in no bound molecules on surface. (b) Tryptophan and (c) Phenylalanine replace WT-Tyrosine in Mutants 2 (M2) and 3 (M3), respectively. The resultant peptides, respectively, are either strongly bound to the surface forming percolated, but finely porous, film (M3) or weakly bound peptides forming isolated islands, each over the course of 3 hours. (d) Fractional coverage trends from time-lapse AFM of WT, M1, M2, and M3; (e) Particle count of each of the peptides; and (f) Average particle size over time; Error bars represent standard deviation of 3 different images from the sample surface, totaling an area of 16 μm2.