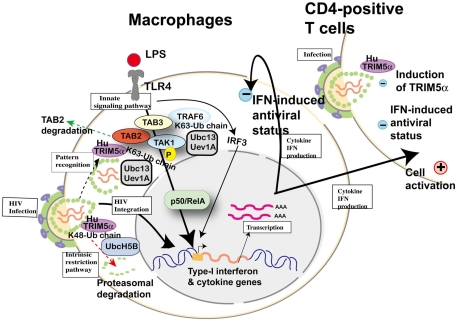
Figure 7.
Cellular factors involved in toll-like receptor (TLR) 4-mediated innate signaling and possible involvement of human TRIM5α in HIV-1 infection. Upon lipopolysaccharide (LPS) stimulation, TLR4 recruits tumor necrosis factor receptor-associated factor 6 (TRAF6) to activate the TGF-β-activated kinase 1 (TAK1) complex (TAK1, TAK1-binding protein (TAB) 2 and TAB3) for NF-κB (p50/RelA heterodimer) activation. TRAF6 is polyubiquitinated by the ubiquitin-conjugating enzyme UBC13–UEV1A. TRIM5α is ubiquitinated by UbcH5B (Xu et al., 2003), but the recognition of HIV-1 core by human TRIM5α and proteasomal degradation (dotted arrow in red) cannot inhibit HIV-1 integration into the human genome. When human TRIM5α recognizes an invasive pathogen (dotted arrow in black), human TRIM5α catalyzes the synthesis of unattached K63-linked ubiquitin chains that activate the TAK1 complex (Pertel et al., 2011). On the other hand, TAB2 is degraded by human TRIM5α (dotted arrow in green; Tareen and Emerman, 2011). Activation of IRF3 and NF-κB-dependent gene expression causes both (+) positive status favorable for viral replication and (−) negative status suppressive for viral replication in macrophages and T cells.