Abstract
Acute renal failure (ARF) is the most frequent and a serious complication in victims of Russell’s viper snakebites. Russell’s viper venom-factor X activator (RVV-X) has been identified as a main procoagulant enzyme involving coagulopathy, which might be responsible for changes in renal hemodynamics and renal functions. Here, we purified RVV-X from crude Russell’s viper venom to study renal hemodynamics, renal functions, intravascular clot, and histopathological changes in Sprague–Dawley rats. Changes in renal hemodynamics and renal functions were evaluated by measuring the mean arterial pressure, glomerular filtration rate (GFR), effective renal plasma flow (ERPF), effective renal blood flow (ERBF), renal vascular resistance (RVR), and fractional excretion of electrolytes. After 10 min, rats receiving both crude venom and purified RVV-X decreased GFR, ERPF, and ERBF and increased RVR. These changes correlated to renal lesions. Along with the determination of intravascular clot, rats injected with purified RVV-X increased the average D-dimer level and reached a peak at 10 min, declined temporarily, and then reached another peak at 30 min. The temporal association between clots and renal dysfunction was observed in rats within 10 min after the injection of purified RVV-X. These findings suggested RVV-X as a major cause of renal failure through intravascular clotting in the renal microcirculation.
Keywords: Daboia russellii siamensis, Russell’s viper venom-factor X activator, Coagulopathy, Renal functions, Renal hemodynamics
1. Introduction
Venomous snakebites are an important medical problem in Southeast Asia. In Thailand, the incidence of snakebites is estimated at 13 per 100,000 persons and the death rate is 0.04 per 100,000 persons (Sangsawang, 2007). Russell’s viper (RV) is the major cause of snakebite morbidity and mortality in many Southeast Asian countries including Thailand, Myanmar, India, Sri Lanka, China, Taiwan, and Indonesia (Warrell, 1989). The subspecies found in Thailand is Daboia russellii siamensis. Symptoms of RV envenoming include edema, pain, and thrombocytopenia with increased risk of systemic bleeding from disseminated intravascular coagulation (DIC). Acute renal failure (ARF) is the main cause of death after an RV bite (Shastry et al., 1977; Sitprija and Boonpucknavig, 1977; Mahasandana et al., 1980; Than et al., 1989; Thein et al., 1991; Tin Nu et al., 1993). Several studies have demonstrated that the pathogenesis of ARF is related to intravascular hemolysis, hypotension, hypovolemia, DIC, and also direct nephrotoxicity of the venom (Chugh et al., 1984; Ratcliffe et al., 1989; Than et al., 1989; Thamaree et al., 1994; Willinger et al., 1995). However, the exact pathogenesis of ARF following D. r. siamensis envenoming is not well established.
Crude RV venom contains several toxins including phospholipase A2 (PLA2), Russell’s viper venom-factor X activator (RVV-X), Russell’s viper venom-factor V activator (RVV-V), proteinases, and other proteins as yet unidentified. Recently, Suwansrinon et al. (2007) studied the effect of RV venom fractions on systemic and renal hemodynamics in dogs. They suggested that the changes in hemodynamics were possibly caused by the proteolytic enzyme in the venom. However, the specific component of RV venom affecting renal hemodynamic alterations remains unclear.
RVV-X is mainly known for its procoagulant property. RVV-X activates coagulation factor X by cleaving a specific peptide bond (Arg52-Ile53) of the heavy chain of the clotting factor, and requires calcium ions for the proteolytic activity (Fujikawa et al., 1972; Di Scipio et al., 1977). As a result, the common coagulation pathway is activated, which leads to the rapid formation of blood clots. Thus, RVV-X should be a major lethal factor in RV venom, which alters renal hemodynamics and renal functions.
In the present study, we demonstrate that RVV-X is the key component that causes renal dysfunction and coagulopathy. Sublethal dose of purified RVV-X replicate renal dysfunction, intravascular clot, and histopathology to that of crude RV venom.
2. Materials and methods
2.1. Venom and antivenom
Lyophilized crude D. r. siamensis venom and commercial horse anti-Russell’s viper F(ab′)2 antivenom (RV antivenom) were obtained from the Queen Saovabha Memorial Institute (QSMI, Thai Red Cross Society, Thailand).
2.2. Purification of RVV-X
Purified RVV-X was purified from lyophilized crude D. r. siamensis venom using open-column gel filtration and anion exchange chromatography, which were modified from Kisiel et al. (1976). Each fraction was screened for factor X activator activity using factor Xa-specific chromogenic substrate S-2765 as described previously (Suntravat et al., 2010). The molecular weight and protein patterns of each fraction were determined by SDS-PAGE and verified by MALDI-TOF mass spectrometry, which was carried out by Bio Service Unit, National Science Technology Development Agency, Thailand.
2.3. Procoagulant activity of purified RVV-X
Procoagulant activity of purified RVV-Xwas tested using the activated partial thromboplastin time assay. A 100 μL of purified RVV-X or crude venom at various concentrations was gently mixed into the tube containing an equal volume of normal human citrated plasma (Instrumentation Laboratory Company, MA, USA) and incubated at 37 °C for 3 min. Then, a pre-warmed 100 μL sample (37 °C) of a 0.025 M CaCl2 was added and the clotting time was recorded. Factor X deficient human plasma (Sigma diagnostics, MO, USA) was used as the negative control substrate for coagulation activity of purified RVV-X. Each test was performed in triplicate.
2.4. Animal experiments
Groups of six male Sprague Dawley rats (200–250 g) were obtained from the National Laboratory Animal Center, Mahidol University, Thailand. Rats were fed with a standard rat diet with water ad libitum. The rats were divided into four groups: (1) crude venom; (2) purified RVV-X; (3) RV antivenom plus purified RVV-X; and (4) normal saline injections. Animals were fasted for 9–10 h prior to each experiment. Each rat was weighed and anesthetized with sodium pentobarbital by intraperitoneal injection (60 mg/kg body weight). Animal protocols were approved by Animal Care and Use Committee of Faculty of Medicine, Chulalongkorn University, Thailand.
2.4.1. Sample injection dosages
The optimum sublethal dose of crude venom (7 μg/kg) was the dose giving no death with abnormal physiological conditions (data not shown). Based on the specific factor X activator activity, the equipotent sublethal dose of purified RVV-X for animal experiments was approximately four times less than that of crude venom. Thus, a 1.75 μg/kg of purified RVV-X was used for an equipotent sublethal dose in rats. RV antivenom completely neutralized factor X activator activity of purified RVV-X using chromogenic substrate S-2765 as the ratio of 250:1. Thus, a 437.5 μg/kg of RV antivenom was used for the neutralizing dose in rats.
2.4.2. Renal hemodynamics and renal functions studies
The studies of renal hemodynamics and renal functions were performed according to the protocol of Yusuksawad and Chaiyabutr (2006). Briefly, a tracheotomy was performed to insert a tracheal tube to open the airway and to remove some secretions. The right common carotid artery was cannulated to collect blood samples and recorded blood pressure with a physiological recorder (Polygraph RM 600, Nihon Kohden Corporation, Japan). The femoral vein was cannulated for sample administration and the solution of inulin and para-aminohippuric acid (PAH). The urinary bladder was exposed and inserted with polyethylene tube for urine collection.
After the surgery, a mixture of 1 g% inulin and 0.2 g% PAH in normal saline was intravenously (i.v.) administrated to the rats at the flow rate of 10 mL/kg body weight/h. Another period of 60 min was allowed for stabilization of the general circulation. Rats groups 1 and 2 were i.v. given a 1 mL of normal saline at the rate of 1 mL/min and immediately followed by a 1 mL of crude venom (7 μg/kg) or purified RVV-X (1.75 μg/kg) at the same injection rate. Rat group 3 was i.v. given a 1 mL of RV antivenom (437.5 μg/kg) within 1 min and immediately followed by a 1 mL of purified RVV-X (1.75 μg/kg) at the same injection rate. Rat group 4 (the control group) was i.v. given normal saline alone in the same manner. Ten minutes after sample injections, two urine samples were collected at 30 and 60 min consecutively. The urine flow rate was measured. A 0.8 mL of blood sample was collected into a 1.5 mL plastic test tube containing 0.8 μL of heparin (5000 IU/mL) at the mid-point of each urine collection. Whole blood samples were collected into two capillary tubes and were centrifuged for 10 min using a microhematocrit centrifuge (Z230H, BHG HERMLE, Germany). Hematocrit values were measured using a micro-capillary reader (IEC, Damon/IEC Division, MA, USA). The remaining heparinized whole blood samples were centrifuged at 2000 g for 10 min at 25 °C to separate the plasma. The inulin concentration in plasma and urine samples was measured by the anthrone method (Burke et al., 1974). The PAH concentration in plasma and urine samples was measured according to the procedure of Yusuksawad and Chaiyabutr (2006). To access glomerular filtration rate (GFR) and effective renal plasma flow (ERPF), the inulin and PAH clearances were calculated according to the formula previously described (Yusuksawad and Chaiyabutr, 2006). The effective renal blood flow (ERBF) was calculated from the measured hematocrit. The renal vascular resistance (RVR) was calculated from the mean arterial pressure and ERBF using the standard formula as previously described (Yusuksawad and Chaiyabutr, 2006). To study the renal tubular function, concentrations of sodium, potassium, chloride, and magnesium in plasma and urine samples were carried out by Renal Laboratory Research Unit, King Chulalongkorn Memorial hospital, Thailand. Urinary excretion and fractional excretion of the electrolytes were determined according to the formula previously described (Yusuksawad and Chaiyabutr, 2006). All groups were compared to the control group, in which rats were i.v. given normal saline alone.
2.4.3. Intravascular clot determination
A blood sample of 0.3 mL was collected and diluted with 3.8% of sodium citrate at 5, 10, 15, 20, 30, 45, 60, 120, and 180 min after sample injections. The D-dimer was determined using the commercial D-Dimer test kit (NycoCard® D-dimer, Axis-Shield PoC, MA, USA). At the end of the intravascular clot experiment, 1 mL of blood was collected and diluted with 1% of EDTA for the determination of the complete blood count using the automated hematology analyzer (Sysmex XS-800i/XS-1000i, Sysmex Corp., Kobe, Japan) at Department of Pediatrics, King Chulalongkorn Memorial hospital, Thailand. Red cell morphology was evaluated on a peripheral blood film. Blood smears were stained with Wright’s stain.
2.5. Histopathological examination
At the end of the intravascular clot experiment, the rat was sacrificed under deep anesthesia. The tissue sections from six rats in each group were determined. Kidneys, adrenal glands, livers, hearts, and lungs were fixed in 10% formalin and stained with hematoxylin and eosin. Histopathological examination was carried out by Special and Molecular Pathology Laboratories, Department of Pathology, King Chulalongkorn Memorial hospital, Thailand.
2.6. Statistical analysis
The results were expressed as mean ± standard deviation. Their significancewas analyzed by the student’s t-test. The level of significance was at P < 0.05. Their significant differences in sample means among groups were analyzed by ANOVA using Least significant difference test as post hoc test at P < 0.05.
3. Results
3.1. Purification of RVV-X and RVV-X activities
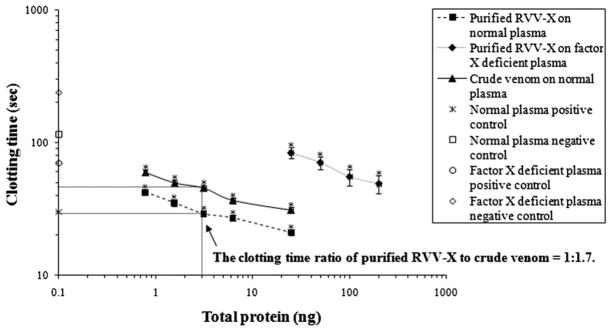
RVV-X was purified from crude venom with approximately a 5% yield. The molecular weight of purified RVV-X was 90,713 Da, which was verified by mass spectrometry. The molecular weight and factor X activator specific activity of purified RVV-X are similar to those of the RVV-X reported by Suntravat et al. (2010). Purified RVV-X significantly shortened the clotting time of normal plasma and factor X deficient plasma in a dose-dependent manner (Fig. 1). The clotting time with 3 ng of purified RVV-Xwas approximately 1.7 times shorter than the clotting time with crude venom.
Fig. 1.
Effects of purified RVV-X and crude venom at various concentrations on clotting time of human normal citrated plasma using the activated partial thromboplastin time assay. The results are expressed as mean + SD (n = 3). An asterisk (*) indicates statistic significance compared with the negative control plasma at P < 0.05. Their significance was analyzed by the student’s t-test. The normal plasma negative control was citrated human plasma reconstituted with only CaCl2. Factor X deficient plasma negative control was factor X deficient human plasma reconstituted with only CaCl2. The positive control was normal citrated or factor X deficient human plasma activated with cephalin (Instrumentation Laboratory Company) and 0.025 M CaCl2.
3.2. Renal hemodynamics and renal functions studies
Effects of crude venom, purified RVV-X, and RV antivenom plus purified RVV-X on renal hemodynamics were summarized in Table 1. Purified RVV-X decreased the mean arterial pressure, GFR and ERPF to the same degree as the equipotent dose of crude venom. Within 10 min of RVV-X and purified RVV-X injections, rats developed significant decreases in GFR, ERPF, and ERBF and significant increase in RVR as compared with the control group. A significant decrease in the mean arterial pressure and significant increase in the filtration fraction was observed in rats injected with crude venom. However, there were no significant differences in all hemodynamic parameters between the crude venom group and the purified RVV-X group.
Table 1.
Renal hemodynamics and renal functions in rats 10 min after injections of crude venom, purified RVV-X, and RV antivenom plus purified RVV-X.
| Group | MAP (mmHg) | GFR (mL/min/100 g BW) | ERPF (mL/min/100 g BW) | ERBF | FF (%) | RVR (mmHg/mL/min/100 g BW) |
|---|---|---|---|---|---|---|
| Crude venom | 95.62 ± 15.05b | 0.43 ± 0.23b | 1.14 ± 0.91b | 1.88 ± 1.46b | 61.45 ± 58.39b | 77.93 ± 26.81b |
| Purified RVV-X | 96.94 ± 10.92ab | 0.50 ± 0.24b | 1.69 ± 0.93b | 2.93 ± 1.63b | 33.46 ± 8.50ab | 81.16 ± 67.60b |
| RV antivenom + purified RVV-X | 107.66 ± 4.43a | 0.77 ± 0.14a | 2.62 ± 0.42a | 4.54 ± 0.67a | 31.25 ± 6.15a | 25.62 ± 3.68a |
| Normal saline | 102.29 ± 8.48ab | 0.82 ± 0.10a | 2.79 ± 0.36a | 4.75 ± 0.62a | 30.00 ± 1.95a | 23.67 ± 3.18a |
The results are expressed as mean ± SD (n = 6).
Abbreviations: MAP, mean arterial pressure; GFR, glomerular filtration rate; ERPF, effective renal plasma flow; ERBF, effective renal blood flow; FF, filtration fraction; RVR, renal vascular resistance.
Statistically significant differences were demonstrated by comparing renal hemodynamics parameters among groups at P < 0.05 (ANOVA).
Values within the same column with different superscripts (a, b) were significantly different at P < 0.05 (ANOVA).
Values followed by the identical superscripts within the same column were not significantly different (P > 0.05).
Purified RVV-X and crude venom envenoming caused renal tubular dysfunction (Table 2). By measuring urine flow rate and urinary excretion of electrolytes, we demonstrated that crude venom and purified RVV-X caused significantly decreases in urine flow rate and urinary excretion of sodium. Urinary excretion of potassium and magnesium was significantly decreased in rats given crude venom. Urinary excretion of chloride was significantly decreased in the purified RVV-X group. However, there were no significant differences in all renal tubular function parameters between the crude venom group and the purified RVV-X group.
Table 2.
Renal tubular functions in rats 10 min after injections of crude venom, purified RVV-X, and RV antivenom plus purified RVV-X.
| Group | V | V/GFR (%) | UNaV (μEq/min/100 g BW) | UKV (μEq/min/100 g BW) | UClV (μEq/min/100 g BW) | UMgV (μEq/min/100 g BW) | FENa (%) | FEK (%) | FECl (%) | FEMg (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Crude venom | 1.67 ± 0.70b | 0.56 ± 0.25a | 0.18 ± 0.08b | 0.34 ± 0.19b | 0.48 ± 0.57ab | 1.28 ± 0.74b | 0.41 ± 0.14ab | 32.46 ± 14.36a | 0.92 ± 0.61a | 18.02 ± 6.99a |
| Purified RVV-X | 2.58 ± 1.35b | 0.48 ± 0.11a | 0.30 ± 0.21b | 0.38 ± 0.22ab | 0.40 ± 0.21b | 1.53 ± 0.63ab | 0.39 ± 0.15ab | 30.16 ± 10.82a | 0.75 ± 0.15a | 16.82 ± 5.20a |
| RV antivenom + purified RVV-X | 4.12 ± 0.90a | 0.52 ± 0.31a | 0.60 ± 0.27a | 0.61 ± 0.16a | 0.82 ± 0.31ab | 2.10 ± 0.50ab | 0.61 ± 0.39b | 28.12 ± 5.98a | 1.03 ± 0.55a | 13.79 ± 4.14a |
| Normal saline | 4.24 ± 0.82a | 0.51 ± 0.06a | 0.70 ± 0.15a | 0.62 ± 0.11a | 0.73 ± 0.11ab | 1.92 ± 0.79ab | 0.58 ± 0.09ab | 29.68 ± 3.50a | 0.80 ± 0.09a | 13.91 ± 5.61a |
The results are expressed as mean ± SD (n = 6).
Abbreviations: V, urine flow rate; V/GFR, fractional excretion of urine; UEV, urinary excretion of electrolytes; FEE, fractional excretion of electrolytes.
Statistically significant differences were determined by comparing renal functions parameters among groups at P < 0.05 (ANOVA).
Values within the same column with different superscripts (a, b) were significantly different at P < 0.05 (ANOVA).
Values followed by the identical superscripts within the same column were not significantly different (P > 0.05).
3.3. Purified RVV-X and crude venom cause intravascular clots
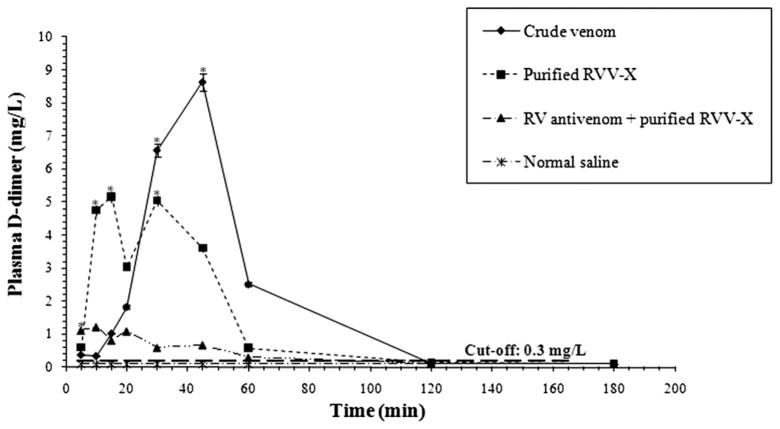
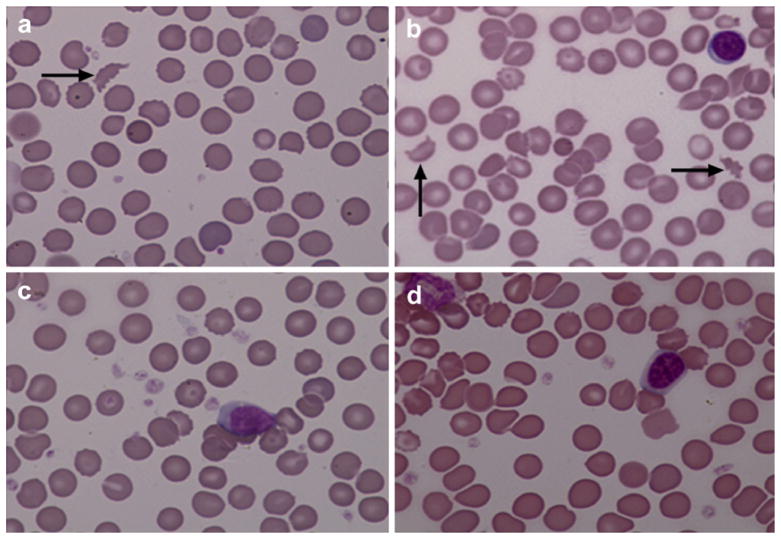
The D-dimer formation is the method of choice for predicting the intravascular clot (Adam et al., 2009). The D-dimer level was serially measured after injections of crude venom and purified RVV-X into rats. Within 10 min of purified RVV-X injection, the average D-dimer level rapidly increased to reach a peak, declined temporarily and then reached another peak at 30 min (Fig. 2). The average D- dimer level of rats given crude venom was gradually increased to reach a peak at 45 min. RV antivenom effectively prevents the D-dimer formation caused by purified RVV-X. Rats given RV antivenom before the injection of purified RVV-X had a minimal increase in the average D-dimer level, which was about 1 mg/L at 5 min and decreased to the baseline at 1 h. The control group did not develop the D-dimer formation. In addition, thrombocytopenia occurred in rats given crude venom and purified RVV-X. Rats given crude venom and purified RVV-X significantly decreased the average platelet count (crude venom = 133 ± 44.08 × 103/μL, P < 0.05; purified RVV-X = 238 ± 136.25 × 103/μL, P < 0.05; the control group = 568 ± 59.84 × 103/μL). Red cell fragments (schistocyte) were found in peripheral blood films of all rats after a 3 h injection of crude venom and purified RVV-X (Fig. 3).
Fig. 2.
Average D-dimer levels in each group of rats at various times. The control group consisted of rats given only normal saline. The results are expressed as mean ± SD (n = 6). An asterisk (*) indicates statistic significance compared with the control group at P < 0.05. Their significance was analyzed by ANOVA using LSD as post hoc test at P < 0.05. A long dashed line indicates the D-dimer cut-off level at 0.3 mg/L.
Fig. 3.
Peripheral blood films (100× magnification) of rats 3 h after injections of (a) crude venom, (b) purified RVV-X, (c) RV antivenom plus purified RVV-X, and (d) normal saline. Blood films were stained with Wright’s stain. The arrow indicates schistocytes.
3.4. Histopathological examination
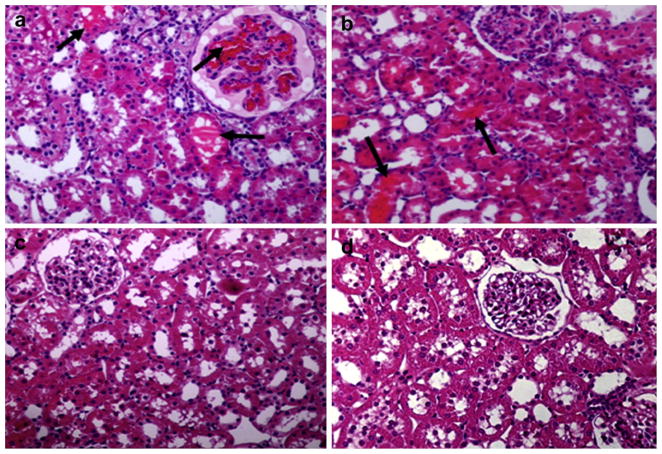
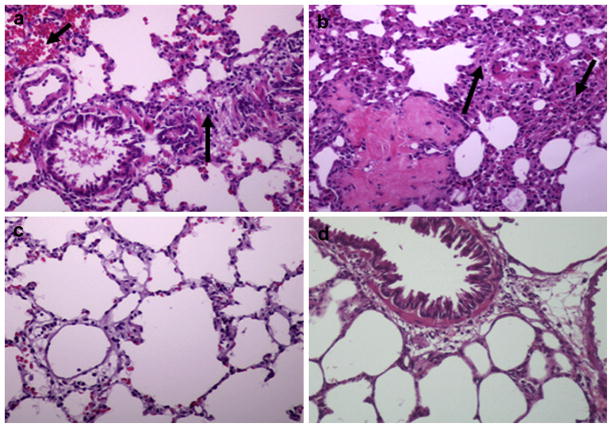
A histopathology study was conducted on kidneys, lungs, adrenal glands, livers, and hearts of six rats in each group; however, Figs. 4 and 5 represent only one rat from each group. We demonstrated fibrin thrombi mainly in kidneys of rats that were injected with crude venom and purified RVV-X (Fig. 4a and b). These rats also had minimal histopathological necrotic changes in the tubular area in kidneys. Inflammatory cell infiltration, intrapulmonary hemorrhage, and vasculitis were clearly observed in the lungs of the same groups of rats (Fig. 5a and b). No histopathological changes in kidneys and lungs were observed in rats receiving RV antivenom plus purified RVV-X and normal saline. Other organs including adrenal glands, livers, and hearts appeared normal (data not shown).
Fig. 4.
Histological sections of kidneys stained with hematoxylin and eosin (20× magnification) of rats 3 h after injections of (a) crude venom, (b) purified RVV-X, (c) RV antivenom plus purified RVV-X, and (d) normal saline. The arrow indicates the fibrin thrombi.
Fig. 5.
Histological sections of lungs stained with hematoxylin and eosin (20× magnification) of rats 3 h after injections of (a) crude venom, (b) purified RVV-X, (c) RV antivenom plus purified RVV-X, and (d) normal saline. The arrow indicates inflammatory cell infiltration and hemorrhage, and vasculitis.
4. Discussion
RVV-X can produce major renal dysfunction, intravascular clot, and fibrin thrombi in kidneys similar to those of crude venom. There was temporal association between clots and renal dysfunction starting within the first 10 min of envenoming. These evidences support our hypothesis that RVV-X would be the key venom component causing renal failure, which was likely due to intravascular clot in the kidney microcirculation rather than direct toxic effect to the kidney.
Hemodynamic changes were possibly caused by the metalloproteinases in the venom (Suwansrinon et al., 2007). Metalloproteinases can activate vasoactive mediators such as TNF-α (Moura-da-Silva et al., 1996). Vasoactive substances have been suggested to correlate with alterations in renal functions and renal hemodynamics (Cumming et al., 1988; Lieberthal et al., 1989; Chan et al., 1994; Lin et al., 2003). The RVV-X, a metalloproteinase, caused changes in renal hemodynamics and renal functions. The change in arterial blood pressure of rats injected with crude venom or purified RVV-X was in the normal range and similar to those observed in the dogs injected with crude venom as previously described (Tungthanathanich et al., 1986; Suwansrinon et al., 2007). The blood pressure of the rats in the experiments is expected to maintain the autoregulation of GFR and ERBF. However, decreases in GFR and ERPF were apparent in the present study. It indicates that purified RVV-X may affect the renal hemodynamics via other factors. Changes in renal hemodynamics in rats induced by purified RVV-X were similar to those changes reported in several studies of crude venom injection (Tungthanathanich et al., 1986; Suwansrinon et al., 2007). Decreases in GFR and ERPF and increases in RVR of rats given crude venom and purified RVV-X were possible that the RVV-X as a protease by itself could cause decreased renal blood flow similar to other enzymes such as metalloproteinase. In addition, the obstruction of renal microvasculature by fibrin thrombi and the glomerular capillary damage in these rats could caused by the procoagulant effect of RVV-X, which might be a contributing factor to hemodynamic changes. These results are supported by the determination of in vitro intravascular clot and the pathological results. The RVV-X could have dual effects on hemodynamics. The highest increase in the average filtration fraction for crude venom was supposed to be attributable to other molecules in crude venom inducing vasoconstriction of the efferent arterioles more than the afferent arterioles in the kidneys (Lonigro et al., 1973; Terragno et al., 1977; Huang, 1984). Changes in renal tubular functions of rats injected with crude venom or purified RVV-X were similar to those observed in the dogs injected with crude venom as previously described (Sanguanrungsirikul et al., 1989; Suwansrinon et al., 2007). Decreases in the urinary excretion of all electrolytes in rats receiving crude venom and purified RVV-X were due to decreased renal blood flow. However, crude venom caused greater reduction in the urinary excretion of sodium than RVV-X suggesting that other venom components such as serine proteases may cause toxicity to the kidneys in addition to RVV-X-induced microangiopathy.
Interestingly, the short-term study of purified RVV-X on intravascular clot had two peaks of the average D-dimer level. The average D-dimer level immediately increased within 10 min and decreased at 20 min. This finding could be caused by the natural clearance of D-dimer by liver. Increased average D-dimer level at 30 min could be due to the platelet activation and consequences of natural clotting process. While crude venom had slower initiation of intravascular clot, which may be due to several components in crude venom including anticoagulants as these are found in other venoms but not yet identified in Russell’s viper venom (Dambisya et al., 1994; Samel et al., 2003). The higher average D-dimer level was probably due to other procoagulants promoting coagulopathy. Previous studies have indicated that D. r. siamensis venom contains RVV-X and RVV-V, which accelerate the clotting of plasma (Hanahan et al., 1972; Kisiel et al., 1976b; Takeya et al., 1992; Gowda et al., 1994). Thrombocytopenia and schistocytes were observed on blood smears of rats given purified RVV-X and crude venom. These results confirm that the intravascular clot occurs after purified RVV-X and crude venominjections.
Histopathologically, minimal changes of tubular necrotic area and some fibrin depositionwere found only in the kidneys of rats receiving crude venom and purified RVV-X. These findings were in accordance with previous reports of victim autopsies (Chugh et al., 1984; Than et al., 1989; Soe et al., 1993). In addition, the inflammation reaction including inflammatory cell infiltration and vasculitis was observed in lungs of the same groups of rats. Similar results of cell infiltration have been observed in Bothrops asper, Bothrops jararaca, Tityus serrulatus, and Androctonus australis hector envenomings (Lomonte et al., 1993; Farsky et al., 1997; Bertazzi et al., 2005; Adi- Bessalem et al., 2008). The activation of pro-inflammatory cytokines, especially TNF-α, IL1-β, and IL-6, plays an important role in the cell recruitment and the activation of mediators resulting in the later inflammatory response (Faccioli et al., 1990; Meki and Mohey El-Dean, 1998; D’Suze et al., 2004). Furthermore, the later inflammatory responses also play an important role in the pathophysiological disturbance (Daisley et al., 1999; Mazzei de Davila et al., 2002; Adi-Bessalem et al., 2008). Interestingly, a decrease in neutrophils in the circulationwas observed in the same groups of rats. These findings correlated to the infiltration of these inflammatory neutrophils in lungs. Neutrophils are usually the first-responder of inflammatory cells to reach the site of inflammation.
We found that Sprague–Dawley rats receiving purified RVV-X had intravascular clots and simultaneously experienced renal dysfunction, which also caused renal pathological changes. This research provides a better understanding of actions of RVV-X on intravascular clot, renal hemodynamics, and renal functions. These findings might lead to the development of new therapeutic strategies.
Acknowledgments
This work was funded by the Royal Golden Jubilee PhD scholarship, the National Research Council of Thailand, and the NNTRC, Texas A&M University-Kingsville: NIH/Viper Resource Center #5 P40 RR018300-08. We gratefully acknowledge all of Snake Bite and Venom Research Unit, Chulalongkorn Medical Research Center, Faculty of Medicine, Chulalongkorn University and the NNTRC staff.
Abbreviations
- ARF
Acute renal failure
- RV
Russell’s viper
- RVV-X
Russell’s viper venom-factor X activator
Footnotes
Ethical statement
This research was approved by Chulalongkorn University Animal Care and Use Committee.
Conflict of interest
None declared.
References
- Adam SS, Key NS, Greenberg CS. D-dimer antigen: current concepts and future prospects. Blood. 2009;113:2878–2887. doi: 10.1182/blood-2008-06-165845. [DOI] [PubMed] [Google Scholar]
- Adi-Bessalem S, Hammoudi-Triki D, Laraba-Djebari F. Pathophysiological effects of Androctonus australis hector scorpion venom: tissue damages and inflammatory response. Exp Toxicol Pathol. 2008;60:373–380. doi: 10.1016/j.etp.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Bertazzi DT, de Assis-Pandochi AI, Talhaferro VL, Caleiro Seixas Azzolini AE, Pereira Crott LS, Arantes EC. Activation of the complement system and leukocyte recruitment by Tityus serrulatus scorpion venom. Int Immunopharmacol. 2005;5:1077–1084. doi: 10.1016/j.intimp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Burke TJ, Navar LG, Clapp JR, Robinson RR. Response to single nephron glomerular filtration rate to distal nephron microperfusion. Kidney Int. 1974;6:230–240. doi: 10.1038/ki.1974.104. [DOI] [PubMed] [Google Scholar]
- Chan L, Chittinandana A, Shapiro JI, Shanley PF, Schrier RW. Effect of an endothelin-receptor antagonist on ischemic acute renal failure. Am J Physiol. 1994;266:F135–F138. doi: 10.1152/ajprenal.1994.266.1.F135. [DOI] [PubMed] [Google Scholar]
- Chugh KS, Pal Y, Chakravarty RN, Datta BN, Mehta R, Sakhuja V, Mandal AK, Sommers SC. Acute renal failure following poisonous snakebite. Am J Kidney Dis. 1984;4:30–38. doi: 10.1016/s0272-6386(84)80023-2. [DOI] [PubMed] [Google Scholar]
- Cumming AD, Driedger AA, McDonald JW, Lindsay RM, Solez K, Linton AL. Vasoactive hormones in the renal response to systemic sepsis. Am J Kidney Dis. 1988;11:23–32. doi: 10.1016/s0272-6386(88)80170-7. [DOI] [PubMed] [Google Scholar]
- Daisley H, Alexander D, Pitt-Miller P. Acute myocarditis following Tityus trinitatis envenoming: morphological and pathophysiological characteristics. Toxicon. 1999;37:159–165. doi: 10.1016/s0041-0101(98)00174-3. [DOI] [PubMed] [Google Scholar]
- Dambisya YM, Lee TL, Gopalakrishnakone P. Action of Calloselasma rhodostoma (Malayan pit viper) venom on human blood coagulation and fibrinolysis using computerized thromboelastography (CTEG) Toxicon. 1994;32:1619–1626. doi: 10.1016/0041-0101(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Di Scipio RG, Hermodson MA, Davie EW. Activation of human factor X (Stuart factor) by a protease from Russell’s viper venom. Biochemistry. 1977;16:5253–5260. doi: 10.1021/bi00643a015. [DOI] [PubMed] [Google Scholar]
- D’Suze G, Salazar V, Diaz P, Sevcik C, Azpurua H, Bracho N. Histopathological changes and inflammatory response induced by Tityus discrepans scorpion venom in rams. Toxicon. 2004;44:851–860. doi: 10.1016/j.toxicon.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Faccioli LH, Souza GE, Cunha FQ, Poole S, Ferreira SH. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration “in vivo” by indirect mechanisms. Agents Actions. 1990;30:344–349. doi: 10.1007/BF01966298. [DOI] [PubMed] [Google Scholar]
- Farsky SH, Walber J, Costa-Cruz M, Cury Y, Teixeira CF. Leukocyte response induced by Bothrops jararaca crude venom: in vivo and in vitro studies. Toxicon. 1997;35:185–193. doi: 10.1016/s0041-0101(96)00135-3. [DOI] [PubMed] [Google Scholar]
- Fujikawa K, Legaz ME, Davie EW. Bovine factor X 1 (Stuart factor). Mechanism of activation by protein from Russell’s viper venom. Biochemistry. 1972;11:4892–4899. doi: 10.1021/bi00776a003. [DOI] [PubMed] [Google Scholar]
- Gowda DC, Jackson CM, Hensley P, Davidson EA. Factor X-activating glycoprotein of Russell’s viper venom. Polypeptide composition and characterization of the carbohydrate moieties. J Biol Chem. 1994;269:10644–10650. [PubMed] [Google Scholar]
- Hanahan DJ, Rolfs MR, Day WC. Observations on the factor V activator present in Russell’s viper venom and its action on factor V. Biochim Biophys Acta. 1972;286:205–211. doi: 10.1016/0304-4165(72)90107-9. [DOI] [PubMed] [Google Scholar]
- Huang HC. Effects of phospholipases A2 from Vipera russelli snake venom on blood pressure, plasma prostacyclin level and renin activity in rats. Toxicon. 1984;22:253–264. doi: 10.1016/0041-0101(84)90026-6. [DOI] [PubMed] [Google Scholar]
- Kisiel W, Hermodson MA, Davie EW. Factor X activating enzyme from Russell’s viper venom: isolation and characterization. Biochemistry. 1976;15:4901–4906. doi: 10.1021/bi00667a023. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Wolf EF, Rennke HG, Valeri CR, Levinsky NG. Renal ischemia and reperfusion impair endothelium-dependent vascular relaxation. Am J Physiol. 1989;256:F894–F900. doi: 10.1152/ajprenal.1989.256.5.F894. [DOI] [PubMed] [Google Scholar]
- Lin YF, Wang JY, Chou TC, Lin SH. Vasoactive mediators and renal haemodynamics in exertional heat stroke complicated by acute renal failure. QJM. 2003;96:193–201. doi: 10.1093/qjmed/hcg029. [DOI] [PubMed] [Google Scholar]
- Lomonte B, Tarkowski A, Hanson LA. Host response to Bothrops asper snake venom. Analysis of edema formation, inflammatory cells, and cytokine release in a mouse model. Inflammation. 1993;17:93–105. doi: 10.1007/BF00916097. [DOI] [PubMed] [Google Scholar]
- Lonigro AJ, Itskovitz HD, Crowshaw K, McGiff JC. Dependency of renal blood flow on prostaglandin synthesis in the dog. Circ Res. 1973;32:712–717. doi: 10.1161/01.res.32.6.712. [DOI] [PubMed] [Google Scholar]
- Mahasandana S, Rungruxsirivorn Y, Chantarangkul V. Clinical manifestations of bleeding following Russell’s viper and Green pit viper bites in adults. Southeast Asian J Trop Med Public Health. 1980;11:285–293. [PubMed] [Google Scholar]
- Mazzei de Davila CA, Davila DF, Donis JH, de Bellabarba GA, Villarreal V, Barboza JS. Sympathetic nervous system activation, antivenin administration and cardiovascular manifestations of scorpion envenomation. Toxicon. 2002;40:1339–1346. doi: 10.1016/s0041-0101(02)00145-9. [DOI] [PubMed] [Google Scholar]
- Meki AR, Mohey El-Dean ZM. Serum interleukin-1beta, interleukin- 6, nitric oxide and alpha1-antitrypsin in scorpion envenomed children. Toxicon. 1998;36:1851–1859. doi: 10.1016/s0041-0101(98)00106-8. [DOI] [PubMed] [Google Scholar]
- Moura-da-Silva AM, Laing GD, Paine MJ, Dennison JM, Politi V, Crampton JM, Theakston RD. Processing of pro-tumor necrosis factor-alpha by venom metalloproteinases: a hypothesis explaining local tissue damage following snake bite. Eur J Immunol. 1996;26:2000–2005. doi: 10.1002/eji.1830260905. [DOI] [PubMed] [Google Scholar]
- Ratcliffe PJ, Pukrittayakamee S, Ledingham JG, Warrell DA. Direct nephrotoxicity of Russell’s viper venom demonstrated in the isolated perfused rat kidney. Am J Trop Med Hyg. 1989;40:312–319. doi: 10.4269/ajtmh.1989.40.312. [DOI] [PubMed] [Google Scholar]
- Samel M, Vija H, Subbi J, Siigur J. Metalloproteinase with factor X activating and fibrinogenolytic activities from Vipera berus berus venom. Comp Biochem Physiol B Biochem Mol Biol. 2003;135:575–582. doi: 10.1016/s1096-4959(03)00171-4. [DOI] [PubMed] [Google Scholar]
- Sangsawang C. Bureau of Epidemiology, Department of Disease Control. Annual Epidemiological Surveillance Report. Ministry of Public Health; Bangkok: 2007. Snake bite; pp. 141–143. [Google Scholar]
- Sanguanrungsirikul S, Chomdej B, Suwanprasert K, Wattanavaha P. Acute effect of Russell’s viper (Vipera russelli siamensis) venom on renal hemodynamics and autoregulation of blood flow in dogs. Toxicon. 1989;27:1199–1207. doi: 10.1016/0041-0101(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Shastry JC, Date A, Carman RH, Johny KV. Renal failure following snake bite. A clinicopathological study of nineteen patients. Am J Trop Med Hyg. 1977;26:1032–1038. doi: 10.4269/ajtmh.1977.26.1032. [DOI] [PubMed] [Google Scholar]
- Sitprija V, Boonpucknavig V. The kidney in tropical snakebite. Clin Nephrol. 1977;8:377–383. [PubMed] [Google Scholar]
- Soe S, Win MM, Htwe TT, Lwin M, Thet SS, Kyaw WW. Renal histopathology following Russell’s viper (Vipera russelli) bite. Southeast Asian J Trop Med Public Health. 1993;24:193–197. [PubMed] [Google Scholar]
- Suntravat M, Nuchprayoon I, Perez JC. Comparative study of anticoagulant and procoagulant properties of 28 snake venoms from families Elapidae, Viperidae, and purified Russell’s viper venomfactor X activator (RVV-X) Toxicon. 2010;56:544–553. doi: 10.1016/j.toxicon.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwansrinon K, Khow O, Mitmoonpitak C, Daviratanasilpa S, Chaiyabutr N, Sitprija V. Effects of Russell’s viper venom fractions on systemic and renal hemodynamics. Toxicon. 2007;49:82–88. doi: 10.1016/j.toxicon.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Takeya H, Nishida S, Miyata T, Kawada S, Saisaka Y, Morita T, Iwanaga S. Coagulation factor X activating enzyme from Russell’s viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J Biol Chem. 1992;267:14109–14117. [PubMed] [Google Scholar]
- Terragno NA, Terragno DA, McGiff JC. Contribution of prostaglandins to the renal circulation in conscious, anesthetized, and laparotomized dogs. Circ Res. 1977;40:590–595. doi: 10.1161/01.res.40.6.590. [DOI] [PubMed] [Google Scholar]
- Thamaree S, Sitprija V, Tongvongchai S, Chaiyabutr N. Changes in renal hemodynamics induced by Russell’s viper venom: effects of indomethacin. Nephron. 1994;67:209–213. doi: 10.1159/000187930. [DOI] [PubMed] [Google Scholar]
- Than T, Francis N, Tin Nu S, Myint L, Tun P, Soe S, Maung Maung O, Phillips RE, Warrell DA. Contribution of focal haemorrhage and microvascular fibrin deposition to fatal envenoming by Russell’s viper (Vipera russelli siamensis) in Burma. Acta Trop. 1989;46:23–38. doi: 10.1016/0001-706x(89)90013-2. [DOI] [PubMed] [Google Scholar]
- Thein T, Tin T, Hla P, Phillips RE, Myint L, Tin Nu S, Warrell DA. Development of renal function abnormalities following bites by Russell’s vipers (Daboia russelii siamensis) in Myanmar. Trans R Soc Trop Med Hyg. 1991;85:404–409. doi: 10.1016/0035-9203(91)90307-k. [DOI] [PubMed] [Google Scholar]
- Tin Nu S, Tin T, Myint L, Thein T, Tun P, Robertson JI, Leckie BJ, Phillips RE, Warrell DA. Renal ischaemia, transient glomerular leak and acute renal tubular damage in patients envenomed by Russell’s vipers (Daboia russelii siamensis) in Myanmar. Trans R Soc Trop Med Hyg. 1993;87:678–681. doi: 10.1016/0035-9203(93)90290-7. [DOI] [PubMed] [Google Scholar]
- Tungthanathanich P, Chaiyabutr N, Sitprija V. Effect of Russell’s viper (Vipera russelli siamensis) venom on renal hemodynamics in dogs. Toxicon. 1986;24:365–371. doi: 10.1016/0041-0101(86)90196-0. [DOI] [PubMed] [Google Scholar]
- Warrell DA. Snake venoms in science and clinical medicine. 1 Russell’s viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83:732–740. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- Willinger CC, Schramek H, Pfaller K, Joannidis M, Deetjen P, Pfaller W. Ultrapure polymerized bovine hemoglobin improves structural and functional integrity of the isolated perfused rat kidney. Ren Physiol Biochem. 1995;18:288–305. doi: 10.1159/000173929. [DOI] [PubMed] [Google Scholar]
- Yusuksawad MS, Chaiyabutr N. Changes in renal hemodynamics in streptozotocin-induced diabetic rats with L-ascorbic acid supplementation. Clin Hemorheol Microcirc. 2006;34:391–399. [PubMed] [Google Scholar]