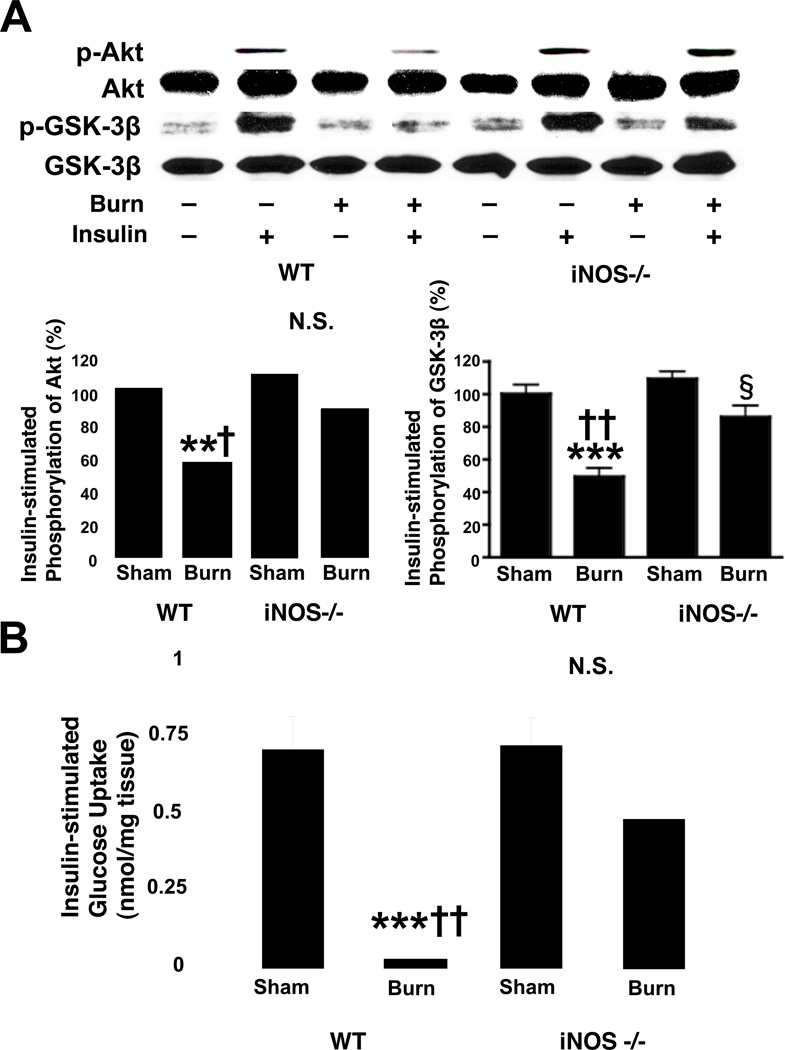
Fig. 4.
Burn injury-induced decreases in insulin-stimulated Akt phosphorylation and glucose uptake in skeletal muscle were reversed by iNOS deficiency. A, At 3 days after burn injury or sham burn, insulin (1 U/kg BW) or saline was injected and 5 min thereafter skeletal muscle was taken. Insulin-stimulated Akt phosphorylation was significantly attenuated by burn injury in wild-type (WT) mice as compared with sham animals. iNOS deficiency (−/−) almost completely reversed decreased insulin-stimulated Akt phosphorylation in burned mice. Similarly, insulin-stimulated GSK-3β phosphorylation was significantly decreased by burn injury in wild-type mice. Burn injury-induced decreased GSK-3β phosphorylation was ameliorated by iNOS deficiency. Neither burn injury nor iNOS deficiency altered Akt or GSK-3β protein expression. The overall interactions between genotype and burn/sham are statistically significant for phosphorylation of Akt (P<0.01) and GSK-3β (P<0.05). **P<0.01, ***p<0.001 vs sham-burned WT, †p<0.05, ††p<0.01 vs burned iNOS −/−, §p<0.05 vs sham-burned iNOS −/−. N.S.: not significant. n=4 per group of animals with saline, n=6 per group of animals with insulin. B, Burn injury resulted in suppression of insulin-stimulated glucose uptake by skeletal muscle ex vivo. iNOS deficiency significantly improved insulin-stimulated glucose uptake in burned mice. The interaction between genotype and burn/sham is statistically significant (P<0.05). ***P<0.001 vs sham-burned WT, ††P<0.01 vs burned iNOS−/−. N.S.: not significant. n=4–6 per group.