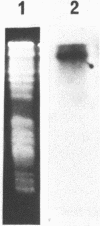
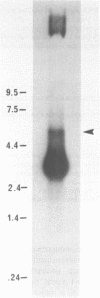
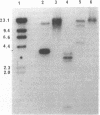
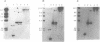Abstract
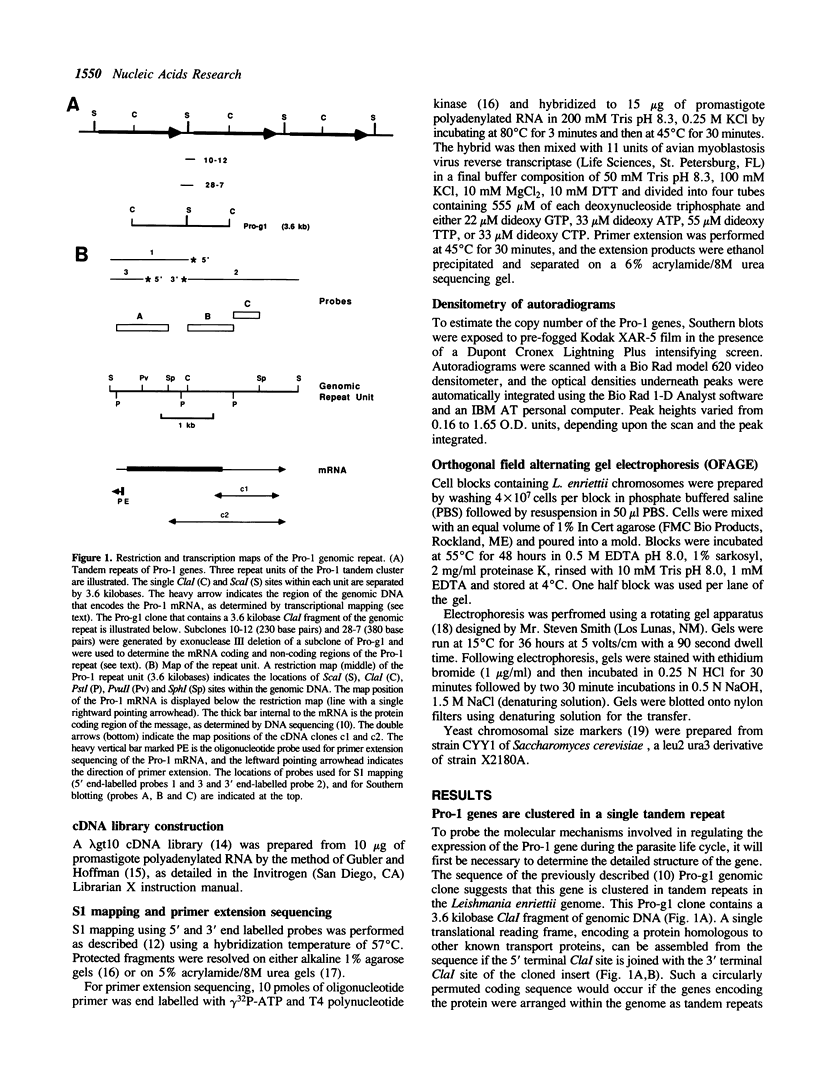
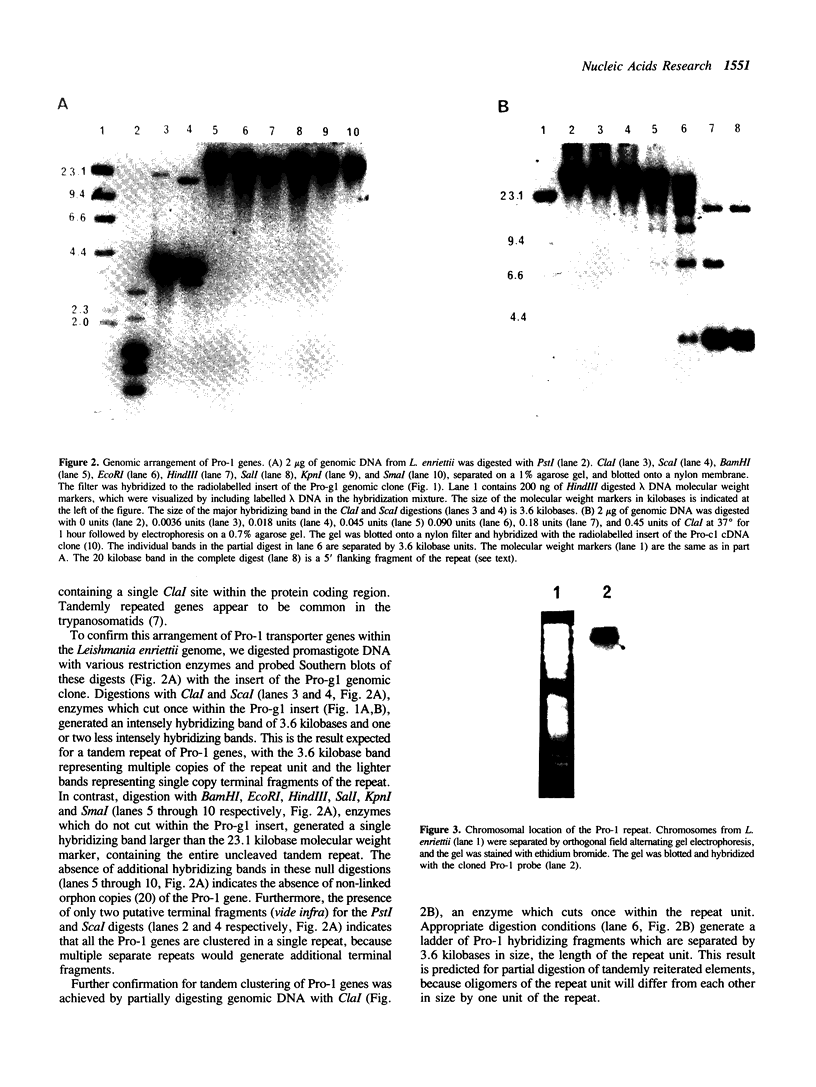
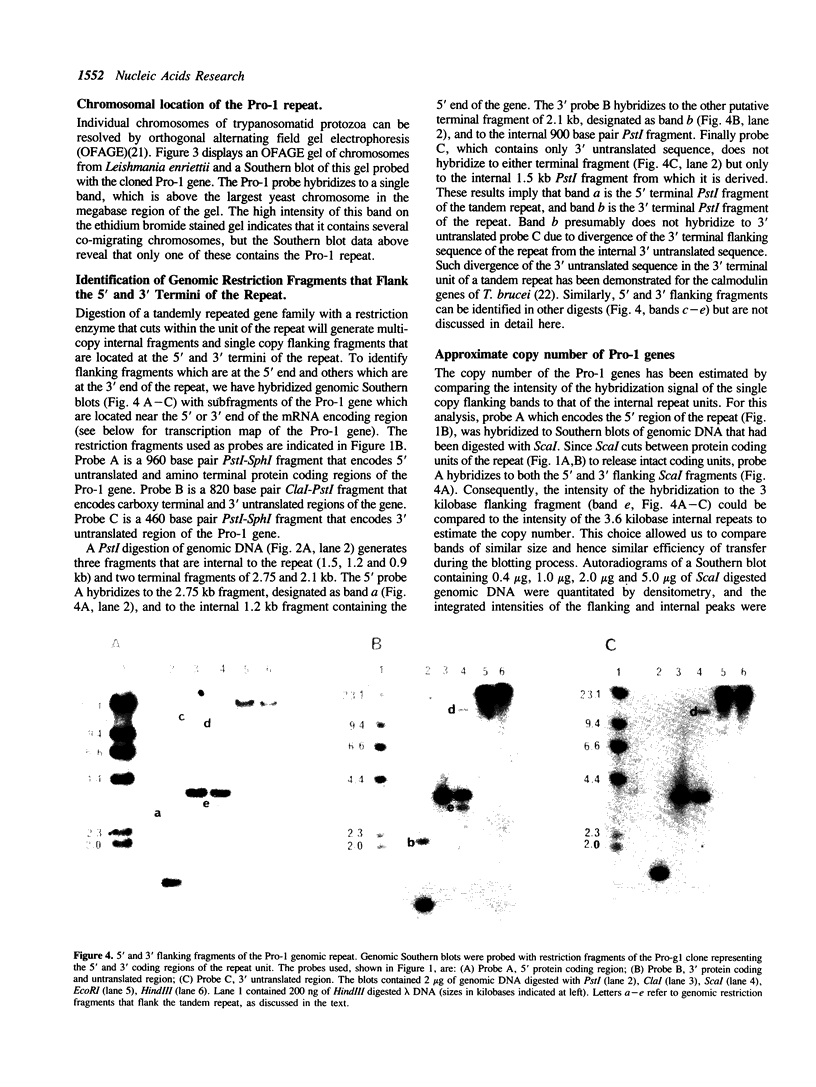
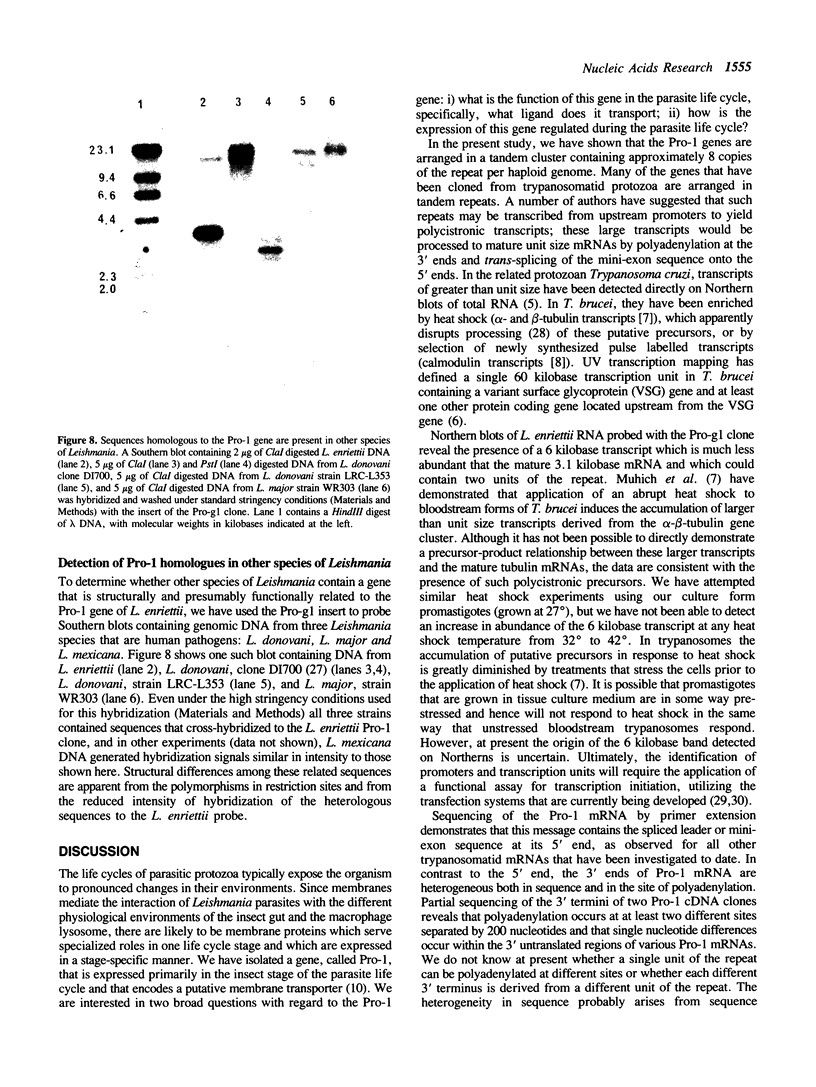
We have previously cloned a gene for a developmentally regulated transport protein from the trypanosomatid protozoan Leishmania enriettii. We demonstrate here that this transporter is encoded by a single family of tandemly clustered genes containing approximately 8 copies of the 3.6 kilobase repeat unit. Transcriptional mapping defines a contiguous 3.3 kilobase region of the repeat unit that encodes the mRNA. The 5' end of the mature mRNA contains the spliced leader or mini-exon previously identified in kinetoplastid protozoa, while the 3' ends of the mRNA are heterogeneous in sequence and in location of the polyadenylation site. We have identified genomic restriction fragments that flank the tandem repeat on the 5' and 3' sides and which may be linked to sequences required for expression of the gene family. Other species of Leishmania also contain sequences that hybridize to the cloned L. enriettii gene at high stringency.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellofatto V., Cross G. A. Expression of a bacterial gene in a trypanosomatid protozoan. Science. 1989 Jun 9;244(4909):1167–1169. doi: 10.1126/science.2499047. [DOI] [PubMed] [Google Scholar]
- Benne R., Van den Burg J., Brakenhoff J. P., Sloof P., Van Boom J. H., Tromp M. C. Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell. 1986 Sep 12;46(6):819–826. doi: 10.1016/0092-8674(86)90063-2. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. An electrophoretic karyotype for yeast. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3756–3760. doi: 10.1073/pnas.82.11.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G., Maxson R., Cohn R. H., Kedes L. Orphons: dispersed genetic elements derived from tandem repetitive genes of eucaryotes. Cell. 1981 Mar;23(3):651–663. doi: 10.1016/0092-8674(81)90428-1. [DOI] [PubMed] [Google Scholar]
- Feagin J. E., Jasmer D. P., Stuart K. Developmentally regulated addition of nucleotides within apocytochrome b transcripts in Trypanosoma brucei. Cell. 1987 May 8;49(3):337–345. doi: 10.1016/0092-8674(87)90286-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Polvere R. I., Van der Ploeg L. H. Conserved sequences and transcription of the hsp70 gene family in Trypanosoma brucei. Mol Cell Biol. 1986 Dec;6(12):4657–4666. doi: 10.1128/mcb.6.12.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Kaur K., Young L., Ullman B. Genetic analysis of nucleoside transport in Leishmania donovani. Mol Cell Biol. 1984 Jun;4(6):1013–1019. doi: 10.1128/mcb.4.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. J., Kooter J. M., Borst P. Inactivation of transcription by UV irradiation of T. brucei provides evidence for a multicistronic transcription unit including a VSG gene. Cell. 1987 Oct 23;51(2):273–281. doi: 10.1016/0092-8674(87)90154-1. [DOI] [PubMed] [Google Scholar]
- Laban A., Wirth D. F. Transfection of Leishmania enriettii and expression of chloramphenicol acetyltransferase gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9119–9123. doi: 10.1073/pnas.86.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear S. M., Wirth D. F. Structure of mRNA encoded by tubulin genes in Leishmania enriettii. Mol Biochem Parasitol. 1985 Apr;15(1):61–82. doi: 10.1016/0166-6851(85)90029-5. [DOI] [PubMed] [Google Scholar]
- Levy S., Sures I., Kedes L. H. Sequence of the 5'-end of Strongylocentrotus purpuratus H2b histone mRNA and its location within histone DNA. Nature. 1979 Jun 21;279(5715):737–739. doi: 10.1038/279737a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Turnbull N. T. Analysis of the 3'-terminal nucleotide sequence of vesicular stomatitis virus N protein mRNA. Nucleic Acids Res. 1978 Nov;5(11):4007–4024. doi: 10.1093/nar/5.11.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Landfear S. M., Wirth D. F. Cloning and characterization of a Leishmania gene encoding a RNA spliced leader sequence. Nucleic Acids Res. 1986 Sep 25;14(18):7341–7360. doi: 10.1093/nar/14.18.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowatt M. R., Clayton C. E. Polymorphism in the procyclic acidic repetitive protein gene family of Trypanosoma brucei. Mol Cell Biol. 1988 Oct;8(10):4055–4062. doi: 10.1128/mcb.8.10.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhich M. L., Boothroyd J. C. Polycistronic transcripts in trypanosomes and their accumulation during heat shock: evidence for a precursor role in mRNA synthesis. Mol Cell Biol. 1988 Sep;8(9):3837–3846. doi: 10.1128/mcb.8.9.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Perry K. L., Watkins K. P., Agabian N. Trypanosome mRNAs have unusual "cap 4" structures acquired by addition of a spliced leader. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8190–8194. doi: 10.1073/pnas.84.23.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Berk A. J., Berget S. M. Transcription maps of adenovirus. Methods Enzymol. 1980;65(1):750–768. doi: 10.1016/s0076-6879(80)65071-x. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Ullu E. Polygene transcripts are precursors to calmodulin mRNAs in trypanosomes. EMBO J. 1988 Feb;7(2):455–463. doi: 10.1002/j.1460-2075.1988.tb02833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschudi C., Young A. S., Ruben L., Patton C. L., Richards F. F. Calmodulin genes in trypanosomes are tandemly repeated and produce multiple mRNAs with a common 5' leader sequence. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3998–4002. doi: 10.1073/pnas.82.12.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]