Abstract
Background
Immunosuppressants decrease circulating dipeptidyl peptidase IV (DPPIV) activity in transplant patients, and decreased DPPIV activity has been associated with angiotensin-converting enzyme (ACE) inhibitor-associated angioedema. One study has reported an increased incidence of ACE inhibitor-associated angioedema among transplant patients compared to published rates, while several case series report angioedema in patients taking specific immunosuppressant agents.
Objective
To test the hypothesis that transplant patients are at increased risk of ACE inhibitor-associated angioedema.
Methods
We assessed the proportion of transplant patients in 145 cases with ACE inhibitor-associated angioedema and 280 ACE inhibitor-exposed controls. We measured the relationship between case–control status, transplant status, and immunosuppressant use and circulating DPPIV activity. We also assessed the incidence of angioedema among consecutive patients who underwent renal or cardiac transplant and were treated with an ACE inhibitor.
Results
Transplant patients were significantly overrepresented among ACE inhibitor-associated angioedema cases compared to controls (odds ratio 18.5, 95% CI 2.3–147.2, P = 0.0004). Immunosuppressant use, chronic renal failure, seasonal allergies and smoking were also associated with ACE inhibitor-associated angioedema in univariate analysis. The association of transplant status with ACE inhibitor-associated angioedema was no longer significant after inclusion of immunosuppressant therapy in a multivariate analysis. Dipeptidyl peptidase IV activity was significantly decreased in sera from cases compared to ACE inhibitor-exposed controls, as well as in individuals taking immunosuppressants. Two of 47 ACE inhibitor-treated renal transplant patients and one of 36 ACE inhibitor-treated cardiac transplant patients developed angioedema.
Conclusion
Transplant patients are at increased risk of ACE inhibitor-associated angioedema possibly because of the effects of immunosuppressants on the activity of DPPIV.
Keywords: angioedema, dipeptidyl peptidase IV, immunosuppressant, transplant
Angiotensin-converting enzyme (ACE) inhibitors reduce morbidity and mortality in patients with congestive heart failure, risk factors for coronary artery disease, and nephropathy (1–3). Rarely, ACE inhibitors cause angioedema, characterized by swelling of the lips, tongue, pharynx, or face (4). Angioedema can lead to airway compromise resulting in intubation, tracheostomy, and even death. The ability to identify patients at increased risk for this side-effect could prevent serious morbidity and even save lives.
The reported incidence of ACE inhibitor-associated angioedema varies from 0.1% to 0.7% calculated from postmarketing surveillance or epidemiologic studies, with higher rates reported in some clinical trials (5–7). Risk of ACE inhibitor-associated angioedema is fourfold to fivefold higher in black Americans compared to white Americans (8, 9). Risk of ACE inhibitor-associated angioedema has also been reported to be increased in women, smokers, and patients with seasonal allergies, but lower in patients with diabetes (7–10). Abbosh et al. (11) have reported a higher-than-expected prevalence of ACE inhibitor-associated angioedema among cardiac and renal transplants. In addition, Duerr et al. (12) reported an increased incidence of angioedema during combined mammalian target of rapamycin (mTOR) inhibitor and ACE inhibitor therapy in kidney transplant recipients. If confirmed, these data suggest that physicians should limit the use of ACE inhibitors in transplant recipients. An association between transplantation and angioedema could also provide insight into the mechanism of ACE inhibitor-associated angioedema.
We report here the association of transplantation with ACE inhibitor-associated angioedema in a study of 145 cases with ACE inhibitor-associated angioedema and 280 ACE inhibitor-exposed controls. We address the possibility of selection bias by conducting a complementary medical record review to ascertain the incidence of angioedema among consecutive kidney and heart transplant recipients at Vanderbilt exposed to an ACE inhibitor. Finally, we propose a possible mechanism underlying the observed association of ACE inhibitor-associated angioedema with immunosuppressant use that of decreased dipeptidyl peptidase IV (DPPIV) activity.
Methods
Identification of cases and controls
The study was approved by the Vanderbilt University Institutional Review Board (IRB), and all subjects provided written informed consent. Cases were identified either at the time of presentation for medical care or by IRB-approved search of the electronic medical record at Vanderbilt University Medical Center using the term ‘angioedema’, as described in detail previously (13). Subjects identified via the electronic record were contacted through their primary care physicians. Individuals were defined as having ACE inhibitor-associated angioedema if they had swelling of the lips, pharynx, or face while on an ACE inhibitor, but had never had angioedema while not taking an ACE inhibitor.
Age-matched controls were also identified by IRB-approved search of the electronic medical record and were defined as individuals who had been treated with an ACE inhibitor for at least 6 months without experiencing angioedema. Based on the anticipated prevalence of black Americans among cases, controls were prespecified to be 50% black Americans and 50% white Americans. We also specified 50% of controls to be women. Medical history, including the history of angioedema, was confirmed by a research nurse or physician using a detailed case report form.
Laboratory analysis
Per protocol, blood for DNA and biochemical assays was collected from cases and controls. Blood was collected in the absence of anticoagulant, centrifuged immediately, and stored at –80°C until assay. Dipeptidyl peptidase IV activity was assayed by incubating sera with a colorimetric substrate, L-glycyl-L-prolyl p-nitroanilide (Sigma, St. Louis, MO, USA), at 37°C, as previously described (14).
Measurement of the effect of immunosuppressant drugs on endothelial cell DPPIV activity in vitro
Human aortic endothelial cells (Invitrogen, Carlsbad, CA, USA) were plated in 96-well dishes and cultured to confluency in Medium-200 (Invitrogen) supplemented with 2% fetal bovine serum, 1 μg/ml hydrocortisone, 10 ng/ml human epidermal growth factor, 3 ng/ml basic fibroblast growth factor, and 10 μg/ml heparin. Confluent cells were treated for 24 h with agents commonly used in transplantation at concentrations similar to therapeutic concentrations in serum. The concentration of sirolimus was 15 ng/ml (16.4 nM), tacrolimus 15 ng/ml (18.7 nM), and cyclosporine A 200 ng/ml (166.3 nM) (all from Sigma) (15). As a vehicle control, cells were treated with 0.01% dimethyl sulfoxyl (DMSO). Cell-surface DPPIV activity was measured using a modification of the assay described earlier. Briefly, adherent cells were washed once with PBS to remove residual media. Cells were then incubated with 1 mM L-glycyl-L-prolyl p-nitroanilide (Sigma) as the substrate in 0.1 M Tris–HCl (pH 8.0) assay buffer for 60 min at 37°C. Dipeptidyl peptidase IV activity was assessed by measuring the rate of change in absorbance at 405 nm. The specificity of the assay on adherent cells was verified with 200 μM Diprotin A (Sigma), a selective DPPIV inhibitor (16, 17).
Retrospective chart review
We conducted an IRB-approved review of the electronic medical record of 320 consecutive renal transplant patients (from January 1, 2005 through July 7, 2007) and 51 consecutive cardiac transplant patients (from January 1, 2005 through February 12, 2008) at Vanderbilt to determine the incidence of angioedema in transplant patients taking an ACE inhibitor.
Statistical analysis
Results are presented as means ± standard deviations. For univariate analysis, continuous variables were compared using Student's t test or the Wilcoxon rank sum test depending on the normality. Discrete variables were compared using either the Chi-square test or Fisher exact test, if one cell contained fewer than 5. Risk factors for ACE inhibitor-associated angioedema were then evaluated by logistic regression. A P-value <0.05 was considered significant. Data were analyzed using spss (Version 15.1; SPSS, Chicago, IL, USA) or SAS for Windows (Version 9; Cary, NC, USA).
Results
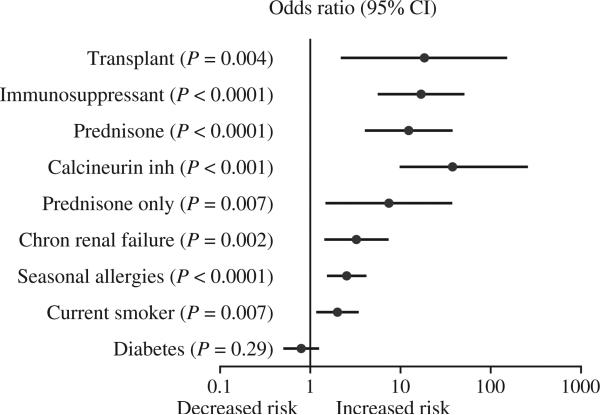
Table 1 provides the clinical characteristics of cases and controls. As reported previously (7, 9), patients with angioedema were more likely to smoke and more likely to have a history of seasonal allergies. There were 145 cases of ACE inhibitor-associated angioedema and 280 ACE inhibitor-tolerant patients. Among the 145 with angioedema, 13 had received a transplant. Four of these cases had received a transplant (one heart, three kidney) after they had presented with ACE inhibitor-associated angioedema (or the temporal sequence was unclear) and were therefore considered nontransplants for the case–control analysis. Nine remaining cases had undergone transplant (three heart, one bilateral lung, six kidney) and were taking immunosuppressant therapy at presentation with ACE inhibitor-associated angioedema. Among the 280 ACE inhibitor-tolerant patients, only one received a transplant. Thus, transplant patients were significantly overrepresented among angioedema cases compared to controls (odds ratio 18.5, 95% CI 2.3–147.2, P = 0.0004). An excess of transplant patients was observed in white Americans with angioedema (six of 71 cases versus none of 143 controls, P = 0.001) but not in black Americans with ACE inhibitor-associated angioedema (three of 74 cases versus one of 135 controls, P = 0.13).
Table 1.
Characteristics of cases and controls
| Angioedema (n = 145) | ACE inhibitor-exposed controls (n = 280) | P value | |
|---|---|---|---|
| Age, years | 57.8 ± 14.2 | 58.1 ± 11.4 | 0.83 |
| Gender, M:F (%)* | 62 (43) : 83 (57) | 142 (51) : 138 (49) | 0.12 |
| Race, B:W:other* | 74 (51) : 71 (49) : 0 | 135 (48) : 143 (51) : 2 (1) | 0.53 |
| Transplant at presentation, N (%) | 9 (6.2) | 1 (0.4) | <0.001 |
| Smokers, N (%)† | 35 (25.7) | 41 (14.8) | 0.007 |
| Diabetes, N (%)† | 47 (32.4) | 105 (37.6) | 0.29 |
| Seasonal allergies, N (%)† | 72 (67.9) | 127 (45.5) | <0.001 |
ACE, angiotensin-converting enzyme.
Mean ± standard deviation unless otherwise noted.
Controls were prespecified to be 50% black Americans and 50% white Americans and 50% female.
Percentage among those subjects for whom data were available.
Table 2 shows the clinical characteristics of the nine patients who had developed angioedema after transplantation and the one control who had received a transplant. All were taking a steroid and a calcineurin inhibitor. An additional 15 individuals (12 cases and three controls) were taking prednisone for the treatment of systemic lupus erythematosus (N = 5), rheumatoid arthritis (N = 3), chronic obstructive lung disease or asthma (N = 4), multiple sclerosis (N = 1), and steroid-responsive arthritis (N = 1). One patient who had not had a transplant was taking cyclosporine for membranous glomerulonephritis. The use of any immunosuppressant, prednisone, or a calcineurin inhibitor was associated with ACE inhibitor-associated angioedema (Fig. 1). Azathioprine use (P = 0.011) and mycophenolate use (P = 0.004) were also associated with ACE inhibitor-associated angioedema; however, because none of the controls were taking these medications it was not possible to calculate an odds ratio.
Table 2.
Characteristics of transplant patients
| Parameter | |
|---|---|
| Race, B:W | 4 : 6 |
| Gender, M:F | 7 : 3 |
| Age, years | 53.4 ± 11.8 |
| Organ transplanted and underlying disease | |
| Heart | Dilated cardiomyopathy, 2 |
| Ischemic cardiomyopathy, 1 | |
| Lung | Cystic fibrosis, 1 |
| Kidney | Polycystic kidney disease, 2 |
| Systemic lupus erythematosus, 1 | |
| Diabetic nephropathy, 1 | |
| Hypertensive nephropathy, 1 | |
| Glomerulonephritis, 1 | |
| Immunosuppressive agents | |
| Steroids, N (%) | 10 (100) |
| Calcineurin inhibitor, N (%) | 10 (100) |
| Azathioprine, N (%) | 3 (30.0) |
| Mycophenolate, N (%) | 3 (30.0) |
| mTOR inhibitor, N (%) | 1 (10.0) |
mTOR, mammalian target of rapamycin.
Figure 1.
Relationship between transplant, immunosuppressant and previously published risk factors and angiotensin-converting enzyme inhibitor-associated angioedema. Data are shown as odds ratio and 95% confidence interval.
To address the possibility that underlying disease may have contributed to the increased risk of ACE inhibitor-associated angioedema associated with transplant or immunosuppressant use, we also assessed the prevalence of chronic renal failure, systemic lupus erythematosus, and rheumatoid arthritis in cases and controls. Chronic renal failure was significantly associated with ACE inhibitor-associated angioedema (Fig. 1), whereas rheumatoid arthritis (P = 0.28) and systemic lupus erythematosus (P = 0.09) were not. In multivariate analysis (Table 3), current transplant was associated with increased risk of ACE inhibitor-associated angioedema, even after controlling for smoking, chronic renal insufficiency, and systemic lupus erythematosus. When immunosuppressant use was added to the model; however, transplant status was no longer independently associated with ACE inhibitor-associated angioedema. Immunosuppressant use and smoking remained significantly associated with angioedema when seasonal allergies were included in the model.
Table 3.
Multivariate logistic regression model for angiotensin-converting enzyme inhibitor-associated angioedema
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Model 1 | |||
| Transplant recipient | 12.42 | 1.47–104.58 | 0.02 |
| Chronic renal failure | 2.83 | 1.18–6.80 | 0.02 |
| Current smoker | 1.99 | 1.18–3.36 | 0.01 |
| Systemic lupus erythematosus | 4.92 | 0.88–27.55 | 0.07 |
| Model 2 | |||
| Transplant recipient | 0.96 | 0.07–12.69 | 0.98 |
| Chronic renal failure | 1.35 | 0.47–3.88 | 0.57 |
| Current smoker | 2.26 | 1.31–3.87 | 0.003 |
| Systemic lupus erythematosus | 0.46 | 0.04–5.07 | 0.53 |
| Immunosuppressant therapy | 20.77 | 4.55–94.93 | <0.0001 |
| Model 3 | |||
| Transplant recipient | 0.5 | 0.03–7.42 | 0.61 |
| Chronic renal failure | 1.86 | 0.58–5.93 | 0.30 |
| Current smoker | 2.28 | 1.24–4.19 | 0.008 |
| Systemic lupus erythematosus | 0.56 | 0.04–7.05 | 0.65 |
| Immunosuppressant therapy | 26.91 | 5.75–125.89 | <0.0001 |
| Seasonal allergies | 2.40 | 1.42–4.07 | 0.001 |
CI indicates confidence interval.
Relationship between case–control or transplant status and DPPIV activity
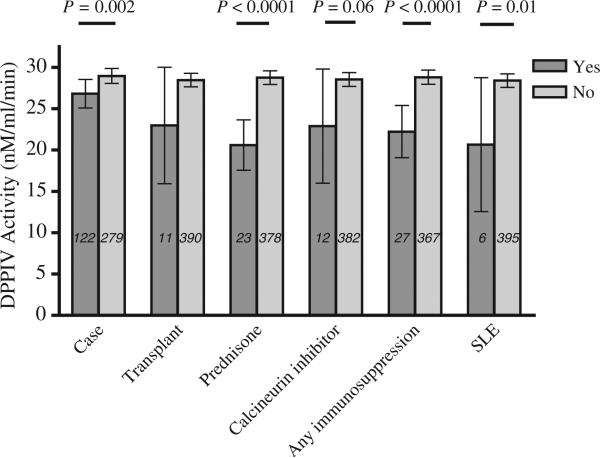
As reported previously (13), DPPIV activity was decreased in the sera of cases who had had ACE inhibitor-associated angioedema compared to controls (P = 0.002) (Fig. 2). Dipeptidyl peptidase IV activity tended to be decreased in transplant patients compared to nontransplants (P = 0.15). Dipeptidyl peptidase IV activity was significantly decreased in individuals with lupus (P = 0.01) or with any autoimmune disease (P = 0.01). Dipeptidyl peptidase IV activity was not significantly different in subjects with and without chronic renal failure (P = 0.41). Dipeptidyl peptidase IV activity was decreased in individuals taking any immunosuppressant (P ≤ 0.0001), taking any prednisone (P < 0.0001), or taking prednisone alone without other immunosuppressant (P = 0.0003) and tended to be decreased in those taking a calcineurin inhibitor (P = 0.07). No patients were taking DPPIV inhibitors.
Figure 2.
Relationship between angiotensin-converting enzyme inhibitor case–control status, transplant status, or immunosuppressant use and dipeptidyl peptidase IV activity. SLE indicates systemic lupus erythematosus. Data are presented as means and 95% confidence intervals.
Retrospective chart review
Three hundred sixteen patients underwent renal transplant from January 1, 2005 through July 7, 2007. One hundred ninety (60.1%) were men. Sixty-five (20.6%) were black Americans, and 241 (76.3%) were white Americans. Forty-seven patients (38 white, eight black, one other) were treated with an ACE inhibitor following transplant. Two of the 47 (4.3%), both white, developed angioedema. None of the renal transplant patients not receiving an ACE inhibitor developed angioedema. Fifty-one patients underwent cardiac transplant from January 1, 2005 through February 12, 2008. Eleven patients (21.6%) were black, 38 (74.5%) were white. Thirty-one (60.8%) were men. Thirty-six (27 white, eight black, one other) were treated with an ACE inhibitor after transplant. One of the ACE inhibitor-treated patients (2.8%), a black female, developed angioedema.
Effect of immunosuppressant drugs on DPPIV activity in cultured endothelial cells
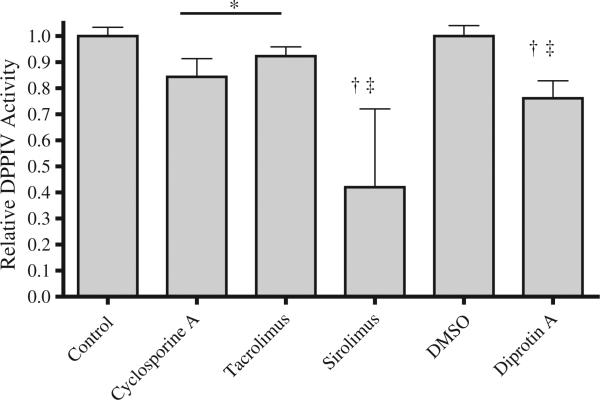
Cultured endothelial cells expressed DPPIV activity, which was inhibited 25% by 24-h treatment with diprotin A, a selective DPPIV inhibitor (Fig. 3) (17). Treatment of the cells with a calcineurin inhibitor, either cyclosporine or tacrolimus, at therapeutic concentrations decreased DPPIV activity 10–15%. The mTOR inhibitor sirolimus decreased DPPIV activity by 60%. There was no effect of prednisone on DPPIV activity in endothelial cells over 24 h (not shown).
Figure 3.
Effect of immunosuppressant agents on dipeptidyl peptidase activity in cultured human aortic endothelial cells. Cells were treated for 24 h, and the concentration of sirolimus was 15 ng/ml (16.4 nM), tacrolimus 15 ng/ml (18.7 nM), and cyclosporine A 200 ng/ml (166.3 nM). DMSO indicates dimethyl sulfoxide. N ≥ 6 for each treatment. *P < 0.05 versus control, †P < 0.005 versus control, ‡P < 0.05 versus the calcineurin inhibitors.
Discussion
We tested the hypothesis that ACE inhibitor-associated angioedema is associated with solid-organ transplant using a case–control study and complementary analysis of the incidence of angioedema among transplant patients exposed to ACE inhibitor at Vanderbilt. We found that transplant recipients were significantly overrepresented among patients with ACE inhibitor-associated angioedema compared to controls. At the same time, the incidence of angioedema among ACE inhibitor-treated patients who had received a kidney (4.3%) or heart (2.8%) transplant was higher than the incidence reported in the general population in the literature.
Abbosh et al. (11) reported previously that five of 105 (4.7%) ACE inhibitor-exposed cardiac transplant patients and one of 91 (1%) ACE inhibitor-treated renal transplant patients developed angioedema. The authors calculated the risk of ACE inhibitor-associated angioedema to be increased 24-fold in cardiac transplant patients and fivefold in renal transplant patients compared to the incidence reported in the literature.
Transplant recipients may be at increased risk of ACE inhibitor-associated angioedema as a result of their underlying disease or as a result of concurrent treatment with immunosuppressant agents. The majority of transplant recipients in the case–control study had underlying renal disease leading to kidney transplantation. Chronic renal insufficiency was associated with increased risk of ACE inhibitor-associated angioedema in univariate analysis but this did not persist in multivariate analysis. Immunosuppressant drug use was also associated with increased risk of angioedema in univariate analysis. The inclusion of immunosuppressant use in multivariate analysis negated the effect of transplant on risk of ACE inhibitor-associated angioedema, suggesting that immunosuppressant use accounted for the observed effect of transplantation.
Angioedema of the bowel has also been reported in an ACE inhibitor-treated patient taking cyclosporine following liver transplant (18). The use of the calcineurin inhibitor tacrolimus or mTOR inhibitors has been associated with the development of angioedema in transplant patients in case series (19–21). Duerr et al. (12) previously reported that nine of 137 (6.6%) of kidney transplant patients who received both mTOR and ACE inhibitors developed angioedema. While in some instances patients were taking ACE inhibitors concurrently, angioedema also occurred in the absence of ACE inhibition. Angioedema was associated with food allergies in children taking tacrolimus or sirolimus (22, 23). In two series, ACE inhibitor-associated angioedema occurred during high-dose, but not during low-dose, tacrolimus or sirolimus (21, 22). The apparent dose-dependent and class-independent effects of immunosuppressant agents on risk of ACE inhibitor-associated angioedema suggest that the mechanism of increased risk relates to the general effect of these agents on lymphocyte activation.
We hypothesize that immunosuppressive agents increase the risk of ACE inhibitor-associated angioedema by decreasing the activity of DPPIV, also known as CD26. Dipeptidyl peptidase IV is a cell-surface marker of T lymphocytes that plays a role in the activation and proliferation of lymphocytes (24). Immunosuppressant agents decrease lymphocyte CD26 expression and, as observed in the present study, circulating DPPIV activity (25). Moreover, inhibiting DPPIV enhances transplant engraftment (26). Our data in cultured endothelial cells suggest that immunosuppressant agents differ in the extent to which they decrease DPPIV activity. Sirolimus was more potent than the calcineurin inhibitors. Consistent with this finding, adding sirolimus to the immunosuppressant regimen of a heart transplant patient, who was also taking an ACE inhibitor, decreased serum DPPIV activity and precipitated angioedema.
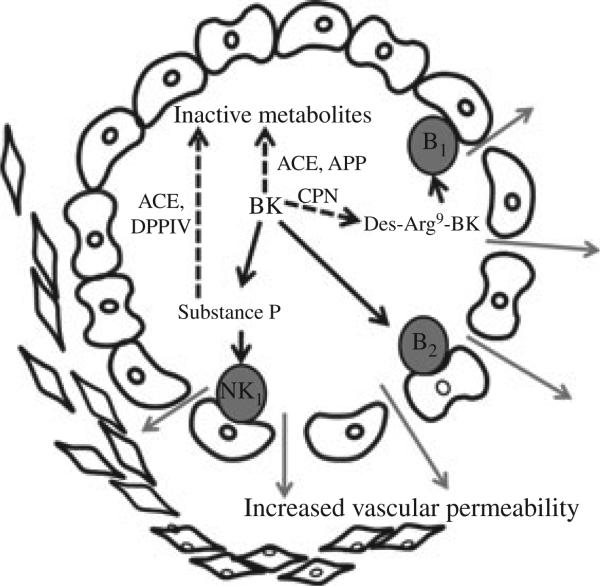
Relevant to the mechanism of angioedema, DPPIV also acts as a peptidase, cleaving the amino-terminal dipeptide from peptides with a penultimate proline or alanine (24). Studies in animal models suggest that ACE inhibitor-associated angioedema results from defective degradation of the vasoactive peptides bradykinin or substance P when ACE is inhibited (27). Bradykinin increases vascular permeability directly by stimulating its B2 receptor and indirectly by stimulating the release of substance P from nerve terminals, which acts to increase vascular permeability via the NK1 receptor (28) (Figure 4). Normally, bradykinin and substance P are degraded primarily by ACE (29). When ACE is inhibited; however, aminopeptidase P inactivates bradykinin, whereas DPPIV inactivates substance P (30, 31).
Figure 4.
Bradykinin increases vascular permeability directly by stimulating its B2 receptor and indirectly by stimulating the release of substance P from nerve terminals. Bradykinin degradation by carboxypeptidase N (CPN) produces an active metabolite, des-Arg9- BK, which stimulates the B1 receptor. Substance P induces vascular permeability by stimulating neurokinin 1 (NK1) receptors. Bradykinin and substance P are degraded by angiotensin-converting enzyme (ACE). During ACE inhibition, bradykinin and substance P are degraded primarily by aminopeptidase P (APP) and dipeptidyl peptidase IV, respectively.
Evidence from studies in animals and in humans implicates DPPIV deficiency in the pathogenesis of ACE inhibitor-associated angioedema. Rats genetically deficient in DPPIV are susceptible to increased peritracheal edema after ACE inhibitor administration, an effect that can be blocked by a substance P (neurokinin 1) receptor antagonist (13). As reported previously in a subset of the current case–control study, we have observed that DPPIV activity is decreased in patients with ACE inhibitor-associated angioedema (10). The recent observation that a pharmacological DPPIV inhibitor increases the risk of ACE inhibitor-associated angioedema provides proof-of-concept for an etiological role of DPPIV in ACE inhibitor-associated angioedema (32). Taken together, these data suggest that environmental factors that decrease DPPIV activity can increase the risk of ACE inhibitor-associated angioedema. It follows then that decreased DPPIV activity during immunosuppression would be associated with increased risk of angioedema. In the case presented in the supplement, the temporal relationship between adjustment of the patient's immunosuppressant regimen, a fall in his circulating DPPIV activity, and the onset of angioedema supports this mechanism.
In conclusion, transplant patients who take an ACE inhibitor are at increased risk of angioedema. We propose that the effect of immunosuppressant agents on DPPIV activity contributes to this increased risk. Physicians, who care for transplant patients, must weigh the risk of angioedema against the benefits of ACE inhibitor use in their patients.
Supplementary Material
Acknowledgments
Funding sources
National Institutes of Health grants HL079184, UL1RR 024975, GM007569.
Abbreviations
- ACE
angiotensin-converting enzyme
- DPPIV
dipeptidyl peptidase IV
- mTOR
mammalian target of rapamycin
Footnotes
To cite this article: Byrd JB, Woodard-Grice A, Stone E, Lucisano A, Schaefer H, Yu C, Eyler AE, Salloum NE, Brown NJ. Association of angiotensin-converting enzyme inhibitor-associated angioedema with transplant and immunosuppressant use.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Case Report: Relationship between angioedema and changes in DPPIV activity and antigen.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald EA, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am. 2006;26:725–737. doi: 10.1016/j.iac.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Slater EE, Merrill DD, Guess HA, Roylance PJ, Cooper WD, Inman WH, et al. Clinical profile of angioedema associated with angiotensin converting-enzyme inhibition. JAMA. 1988;260:967–970. [PubMed] [Google Scholar]
- 6.Sica DA. The African American Study of Kidney Disease and Hypertension (AASK) trial: what more have we learned? J Clin Hypertens (Greenwich) 2003;5:159–167. doi: 10.1111/j.1524-6175.2003.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Brown NJ, Ray WA, Snowden M, Griffin MR. Black americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60:8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- 9.Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- 10.Byrd JB, Touzin K, Sile S, Gainer JV, Yu C, Nadeau J, et al. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51:141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbosh J, Anderson JA, Levine AB, Kupin WL. Angiotensin converting enzyme inhibitor-induced angioedema more prevalent in transplant patients. Ann Allergy Asthma Immunol. 1999;82:473–476. doi: 10.1016/S1081-1206(10)62723-8. [DOI] [PubMed] [Google Scholar]
- 12.Duerr M, Glander P, Diekmann F, Dragun D, Neumayer HH, Budde K. Increased Incidence of Angioedema with ACE Inhibitors in Combination with mTOR Inhibitors in Kidney Transplant Recipients. Clin J Am Soc Nephrol. 2010;5:703–708. doi: 10.2215/CJN.07371009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JB, Shreevatsa A, Putlur P, Foretia D, McAlexander L, Sinha T, et al. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol. 2007;120:403–408. doi: 10.1016/j.jaci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre J, Murphey LJ, Hartert TV, Jiao SR, Simmons WH, Brown NJ. Dipeptidyl peptidase IV activity in patients with ACE-inhibitor-associated angioedema. Hypertension. 2002;39(2 Pt 2):460–464. doi: 10.1161/hy0202.103054. [DOI] [PubMed] [Google Scholar]
- 15.Buron F, Perrin H, Malcus C, Hequet O, Thaunat O, Kholopp-Sarda MN, et al. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplant Proc. 2009;41:3347–3352. doi: 10.1016/j.transproceed.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Gabrilovac J, Abramic M, Uzarevic B, Andreis A, Poljak L. Dipeptidyl peptidase IV (DPPIV) enzyme activity on immature T-cell line R1.1 is down-regulated by dynorphin-A(1-17) as a non-substrate inhibitor. Life Sci. 2003;73:151–166. doi: 10.1016/s0024-3205(03)00257-1. [DOI] [PubMed] [Google Scholar]
- 17.Qi SY, Riviere PJ, Trojnar J, Junien JL, Akinsanya KO. Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases. Biochem J. 2003;373(Pt 1):179–189. doi: 10.1042/BJ20021914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg EI, Mishra G, Abdelmalek MF. Angiotensin-converting enzyme inhibitor-induced isolated visceral angioedema in a liver transplant recipient. Transplantation. 2003;75:730–732. doi: 10.1097/01.TP.0000048491.67462.DA. [DOI] [PubMed] [Google Scholar]
- 19.Mahe E, Morelon E, Lechaton S, Kreis H, de PY, Bodemer C. Angioedema in renal transplant recipients on sirolimus. Dermatology. 2007;214:205–209. doi: 10.1159/000099584. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs U, Zittermann A, Berthold HK, Tenderich G, Deyerling KW, Minami K, et al. Immunosuppressive therapy with everolimus can be associated with potentially life-threatening lingual angioedema. Transplantation. 2005;79:981–983. doi: 10.1097/00007890-200504270-00020. [DOI] [PubMed] [Google Scholar]
- 21.Stallone G, Infante B, Di PS, Schena A, Grandaliano G, Gesualdo L, et al. Sirolimus and angiotensin-converting enzyme inhibitors together induce tongue oedema in renal transplant recipients. Nephrol Dial Transplant. 2004;19:2906–2908. doi: 10.1093/ndt/gfh352. [DOI] [PubMed] [Google Scholar]
- 22.Lykavieris P, Frauger E, Habes D, Bernard O, Debray D. Angioedema in pediatric liver transplant recipients under tacrolimus immunosuppression. Transplantation. 2003;75:152–155. doi: 10.1097/00007890-200301150-00027. [DOI] [PubMed] [Google Scholar]
- 23.Ozdemir O, rrey-Mensah A, Sorensen RU. Development of multiple food allergies in children taking tacrolimus after heart and liver transplantation. Pediatr Transplant. 2006;10:380–383. doi: 10.1111/j.1399-3046.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 24.Lambeir AM, Durinx C, Scharpe S, De MI. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 25.Scharpe S, DeMeester I, Vanhoof G. Serum dipeptidyl peptidase IV activity in transplant recipients. Clin Chem. 1990;36:984. [Google Scholar]
- 26.Korom S, De MI, Stadlbauer TH, Chandraker A, Schaub M, Sayegh MH, et al. Inhibition of CD26/dipeptidyl peptidase IV activity in vivo prolongs cardiac allograft survival in rat recipients. Transplantation. 1997;63:1495–1500. doi: 10.1097/00007890-199705270-00021. [DOI] [PubMed] [Google Scholar]
- 27.Emanueli C, Grady EF, Madeddu P, Figini M, Bunnett NW, Parisi D, et al. Acute ACE inhibition causes plasma extravasation in mice that is mediated by bradykinin and substance P. Hypertension. 1998;31:1299–1304. doi: 10.1161/01.hyp.31.6.1299. [DOI] [PubMed] [Google Scholar]
- 28.Campos MM, Calixto JB. Neurokinin mediation of edema and inflammation. Neuropeptides. 2000;34:314–322. doi: 10.1054/npep.2000.0823. [DOI] [PubMed] [Google Scholar]
- 29.Skidgel RA. Characterization of the metabolism of substance P and neurotensin by human angiotensin I converting enzyme and “enkephalinase”. Prog Clin Biol Res. 1985;192:371–378. [PubMed] [Google Scholar]
- 30.Ward PE, Chow A, Drapeau G. Metabolism of bradykinin agonists and antagonists by plasma aminopeptidase P. Biochem Pharmacol. 1991;42:721–727. doi: 10.1016/0006-2952(91)90028-4. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidas IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther. 1992;260:1257–1261. [PubMed] [Google Scholar]
- 32.Brown NJ, Byiers S, Carr D, Maldonado M, Warner BA. Dipeptidyl peptidase-IV inhibitor use associated with increased risk of ACE inhibitor-associated angioedema. Hypertension. 2009;54:468–470. doi: 10.1161/HYPERTENSIONAHA.109.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.