Figure 2. Laboratory analysis of XP3HM cells.
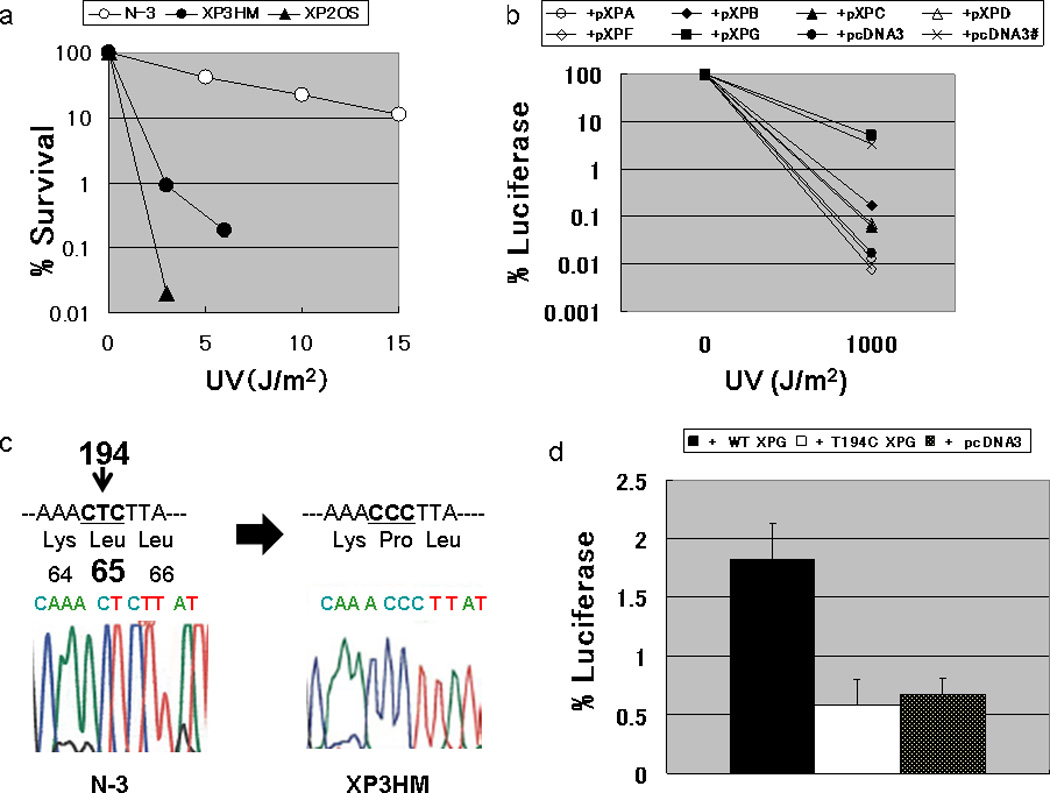
(a) Post-UV survival of the cells: The D0 value (a UV dose that results in 37% cell survival) of XP3HM cells (0.6 J/m2) was much lower than that of the normal N-3 cells (D0 value; 5.0 J/m2). However, these cells were less sensitive than XP2OS (XP-A) (D0 value; 0.3 J/m2). (b) Host cell reactivation assay for the assignment of XP complementation group G in XP3HM cells: The cells were co-transfected with a UV-damaged luciferase gene expression vector along with expression vectors harboring cloned wild-type XP cDNA. Increased luciferase activity was observed when the wild type XPG cDNA expression plasmid was transfected into the cells from the patient, while luciferase activity was still very low after the transfection of expression vectors harboring other XP cDNA (wt XPA, XPB, XPC, XPD, XPF) or the empty vector (pcDNA3). The restored DNA repair capacity after transfecting the wild type XPG cDNA into the XP3HM cells reached the level observed in normal cells (x) after transfecting the empty vector (pcDNA3)., (c) Nucleotide sequence analysis of the XPG gene in XP3HM cells: We identified a homozygous T to C change in exon 2 of the XPG cDNA (c.194T>C) with predicted amino acid change (p.L65P). (d) Host cell reactivation assay for analysis of the DNA repair function of the c.T194C mutation in exon 2 of the XPG gene. We constructed a pXPGT194C plasmid using a pXPG plasmid and QuikChange Site-Directed Mutagenesis Kit (Stratagene, CA, USA). After the transfection of pXPGT194C into the XP20BE cells, the luciferase activity (or the DNA repair capacity; DRC) was not significantly different from the control empty vector plasmid (pcDNA3), while the wild type XPG cDNA increased the DRC of the patient’s cells.
