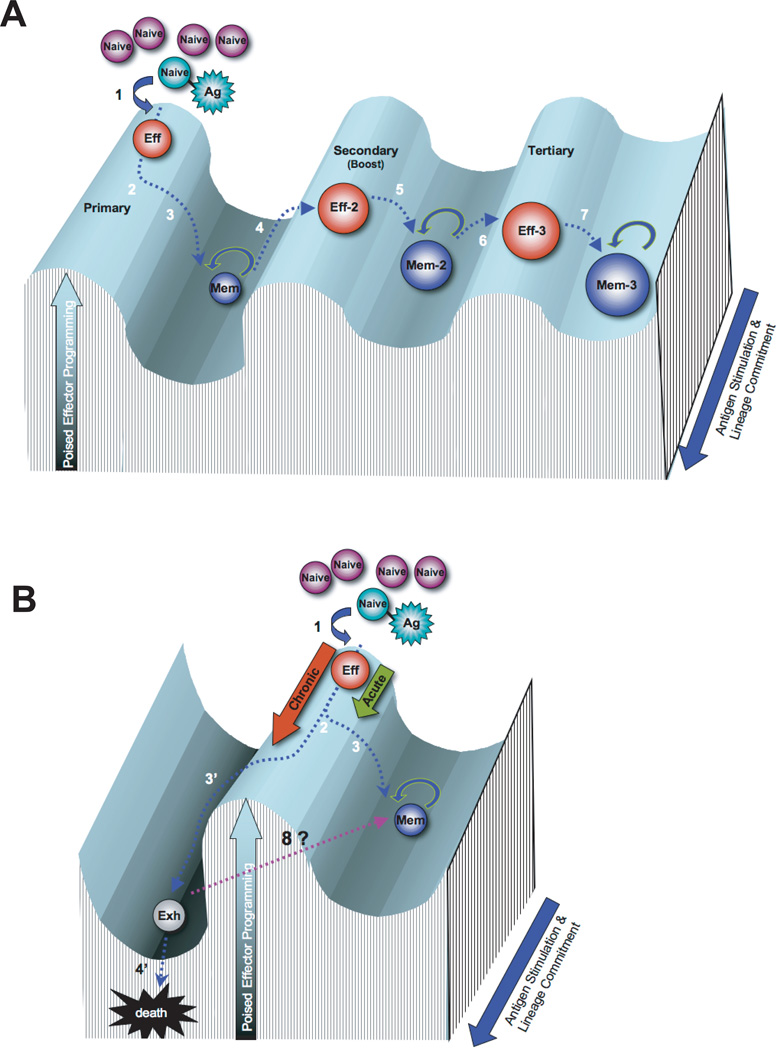
Figure 1.
Rolling hill diagram of antigen-mediated transcriptional reprogramming of CD8 T cells during memory differentiation. A) Differentiation of antigen-specific CD8 T cells during primary, secondary, and tertiary acute viral infection. B) Differentiation of antigen-specific CD8 T cells during chronic viral infection versus acute viral infection. The peaks and troughs of the transcriptional landscape of the Y-axis represent the potential to achieve an effector response through the modification of transcriptional programs. The Z-axis (blue arrow) represents the duration of antigen exposure and the lineage commitment of the antigen-specific CD8 T cell. Step 1: Antigen-specific naïve CD8 T cells encounter an antigen-presenting cell and differentiate into cytolytic effector cells. Step 2: During the ~ 105 fold expansion of effector cells a subset of cells commit to a memory fate. Step 3: Following antigen clearance the effector population contracts, with ~5–10% of the population surviving to differentiate into resting memory CD8 T cells, poised to rapidly recall the effector functions. Step 4: Memory CD8 T cells efficiently recall an effector transcriptional profile upon secondary exposure to the antigen or a vaccine boost regimen. Step 5: Secondary effector CD8 T cells undergo less contraction resulting in a greater quantity of self-renewing memory CD8 T cells with a gene expression profile that retains some effector-like transcriptional status. Steps 6 & 7: The resulting memory population generated from a tertiary infection are more effector-like and increased in quantity relative to the primary and secondary memory cells. Step 3’: If the infection persists the antigen-specific CD8 T cells further differentiate with progressive restriction in the ability to recall effector functions and ultimately loss of the virus-specific cells. Step 8: Future efforts will determine if exhausted CD8 T cells can be reprogrammed to obtain the transcriptional regulation of a resting memory CD8 T cell.