Abstract
Because of the pathological role of IL-6 in rheumatoid arthritis (RA), tocilizumab (TCZ), a humanized anti-IL-6 receptor monoclonal antibody, was expected to improve inflammation and joint destruction of RA. Indeed, randomized clinical trials demonstrated the clinical efficacy of TCZ as monotherapy or combined with methotrexate (MTX) for RA patients with inadequate responses to disease-modifying antirheumatic drugs, MTX or tumor necrosis factor (TNF) inhibitors. Although long-term tolerability for TCZ is superior to that for TNF inhibitors, information regarding the potency of drug free remission of TCZ is limited at present. In terms of its safety profile, the general risk of infection when using TCZ is comparable to that of TNF inhibitors. TCZ has some advantage in RA patients who can not use MTX and are non-responders to TNF inhibitors. In conclusion, TCZ is one of the most prospective next generation biologics for the treatment of RA.
Keywords: rheumatoid arthritis, interleukin-6, tocilizumab
Introduction
Interleukin (IL)-6 was originally cloned as a B cell differentiation factor that promotes B cell differentiation into antibody-producing cells.1 Subsequent in vitro studies and analysis of IL-6 transgenic mice have shown that IL-6 acts not only on B cells but also on T cells, hepatocytes, hematopoietic progenitor cells and various other cells.2–4 One of the important functions of IL-6 is induction of the differentiation of CD4+ naïve T cells into effector cells. IL-6 promotes naïve T cell differentiation into Th17 cells in the presence of transforming growth factor (TGF)-β, while it inhibits TGF-β-induced regulatory T cell (Treg) differentiation.5 IL-6 is also reported to be able to induce the switching of Treg into Th17 cells. An imbalance between Th17 and Treg is considered to be the primary pathogenesis of several autoimmune diseases.6 Based on these findings, it was anticipated that IL-6 blockage would constitute a novel treatment strategy for autoimmune and inflammatory diseases.4,7–9 Tocilizumab (TCZ) was developed to this end. TCZ is a humanized anti- IL-6 receptor (IL-6R) monoclonal antibody (Ab) of the IgG1 class that was generated by grafting the complementarity-determining regions of a mouse anti-human IL-6R Ab onto human IgG1. TCZ blocks all IL-6-mediated signals by inhibiting IL-6 binding to transmembrane and soluble IL-6Rs.
Mechanism of Action, Metabolism and Pharmacokinetic Profile
Rheumatoid arthritis (RA) is a chronic, progressive inflammatory disease of the joints and surrounding tissues accompanied by intense pain, irreversible joint destruction and systemic complications such as fatigue, anemia and fever.10 Within joints, inflammatory cells invade the synovium, which is accompanied by neovascularization. Synoviocyte hyperplasia then forms synovitis, the so-called pannus. Activated synovitis causes destruction of cartilage and erosion of the adjacent bone. Increased IL-6 levels in the sera and synovium of RA patients and proinflammatory activities of IL-6 such as augmentation of synovial fibroblast proliferation, osteoclast differentiation, and the production of matrix metalloproteinase (MMP) and vascular endothelial growth factor (VEGF) indicate that IL-6 is involved in the pathogenesis of RA.11–16
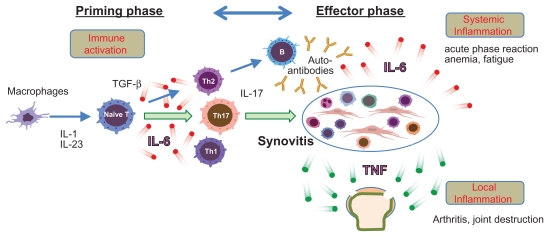
Induction of type II collagen-induced arthritis (CIA) requires CD4 T cells and leads to the production of anti-type II collagen IgG. The incidence and severity of arthritis of CIA was reduced following IL-6 blockade, which was achieved by gene knockout or by administration of an anti-IL-6R Ab.17–22 In contrast, the arthritis of another arthritis model termed antitype II collagen antibody-induced arthritis (CAIA) has skipped the priming phase of T cell dependent antibody generation. Although IL-6 is elevated in both models, CAIA was profoundly suppressed in tumor necrosis factor (TNF) −/− mice but not in IL-6−/− mice, indicating that TNF might play a more significant role in the development of CAIA than IL-6.23 This observation suggests that, in this model, IL-6 is required for activation of T cell responses and for the production of antibodies specific for joint components (priming phase) and that TNF is necessary for the generation of arthritis (effector phase).24 We found that TCZ was not effective for two patients with psoriatic arthritis.25 This type of arthritis, unlike rheumatoid arthritis, does not appear to require immune activation for its development. Figure 1 shows the pathologic role of IL-6 in the development of RA. IL-6 contributes to immune activation of RA and systemic inflammation while TNF mainly contributes to arthritis and joint destruction.
Figure 1.
The pathological role of IL-6 in RA.
Notes: The pathogenesis of rheumatoid arthritis is composed of a priming phase and an effector phase. In the priming phase, IL-6-induced Th17 development and the induction of autoantibodies such as rheumatoid factor (RF) are important. Th17-dependent immune activation generates active synovitis. Synovitis is an epicenter of the inflammation of RA. Systemic inflammation (production of acute phase protein, anemia and fatigue) is mainly mediated by IL-6. Local inflammation (arthritis) such as arthralgia, swelling, and joint destruction is mainly mediated by TNF.
Recommended TCZ dosing regimens differ depending on the area. In European countries and Japan TCZ is administered intravenously at a dose of 8 mg/kg every 4 weeks. In the USA, it is recommended that TCZ should be initially administered at a dose of 4 mg/kg every 4 weeks and it is then permitted to increase the dose up to 8 mg/kg every 4 weeks if clinically indicated. At steady state, the elimination half-life of TCZ at a dose of 4 mg/kg and 8 mg/kg is 11 days and 13 days, respectively.26 Serum TCZ levels of more than 1 μg/mL are recommended to maintain sufficient efficacy. The level of serum CRP is a good marker for titration of TCZ. Thus, if serum concentrations of TCZ exceed 1 μg/mL at the trough level, this means that IL-6 function is blocked in vivo and therefore CRP levels are within the normal range. An increase in CRP level suggests that inhibition of IL-6 function by TCZ is not complete. Although at the present time it is not indicated, dose-up or interval shortening of TCZ administration might produce better outcome. TCZ administration at 2-week intervals is approved in Japan for treatment of Castleman’s disease and systemic juvenile idiopathic arthritis, in which a large amount of IL-6 in the sera was found. In addition to its effect on the amount of IL-6 production, the rate of TCZ degradation also influences the efficacy of TCZ. We found that more TCZ is required when patients have splenomegaly.27 Studies of TCZ tissue distribution in cynomolgus monkeys demonstrated that high levels of TCZ remain not only in synovial fluid and plasma but also in spleen for up to 28 days after TCZ administration. Excessive accumulation of TCZ in spleen may reduce the anti-rheumatic effect of TCZ.
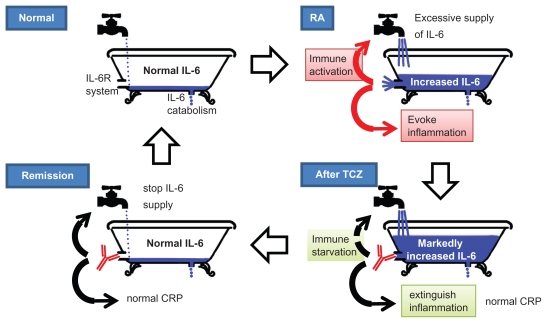
During TCZ therapy, the serum IL-6 concentration temporarily increases. The so-called “bathtub theory”, as shown in Figure 2, illustrates this phenomenon.28 TCZ does not directly inhibit the production of IL-6. As long as free TCZ is detectable, soluble IL-6R is saturated with TCZ and IL-6 signaling is completely inhibited. Free serum IL-6 increases because IL-6R mediated binding of IL-6 is inhibited by the unavailability of TCZ-free IL-6R. Although systemic inflammation is immediately blocked by TCZ, IL-6 production is not inhibited until immune stabilization is induced by TCZ. Normalization of serum IL-6 level suggests repair of the immunological abnormality and indeed clinical remission is achieved in such RA patients.
Figure 2.
Mechanism of action of tocilizumab in RA (bathtub theory).
Notes: The mechanism of tocilizumab (TCZ) in rheumatoid arthritis was illustrated as the bathtub theory by Nishimoto et al.28 The faucet (immune cells) supplies IL-6 into the bathtub (blood). Under normal conditions, the supplied IL-6 is completely cleared by soakaway (catabolism), there is no IL-6 overflow into the IL-6 receptor system (drain) and there is no IL-6 function. In RA, the faucet is fully opened and soakaway cannot completely eliminate the increased production of IL-6. Therefore IL-6 overflows into the drain and IL-6 acts pleiotropically. This IL-6 induces immune activation and systemic inflammation through the IL-6R system. After TCZ therapy, the drain is blocked. However, IL-6 is not eliminated from the blood. Although the IL-6 cannot act through its receptors, the serum IL-6 level is increased because soakaway cannot completely eliminate IL-6. Thus, IL-6 function is blocked by TCZ and inflammation is suppressed. Immune starvation by TCZ gradually closes the faucet (clinical remission). The IL-6 supply is stopped and serum IL-6 levels revert to normal. Compete starvation of immune activation may lead to cessation of TCZ treatment (drug-free remission).
Phase I-III Clinical Trials
Phase I and II clinical trials of TCZ have been performed and were reported between 2002 and 2006. Summaries of phase I/II trials are shown in Table 1. The first trial was performed in the UK29 using single administration of a placebo or TCZ at a dosage of 0.1, 1, 5 or 10 mg/kg. Patients who were treated with 5 mg/kg or 10 mg/kg of TCZ showed significant improvement at week 2. After pharmacokinetic assessment,30 a study for dose setting trial was performed in Japan.31 Patients were administered TCZ (4 or 8 mg/kg) monotherapy or placebo every 4 weeks. Both doses of TCZ were effective but the 8 mg/kg dose group showed a greater improvement than the 4 mg/kg dose group.
Table 1.
Phase I and II clinical trials of tocilizumab.
| Study | Population studied | Administration duration | N | Treatment arm | Conclusion | Ref. |
|---|---|---|---|---|---|---|
| Phase I (UK) | DMARDs-IR | 2 weeks | 45 | 0.1, 1, 5, 10 mg/kg, TCZ or PBO | Well tolerated and efficacious at doses of 5 and 10 mg/kg TCZ | 29 |
| Phase I/II (Japan) | 6 weeks | 15 | TCZ (8 mg/kg) monotherapy | Well tolerated and efficacious | 30 | |
| Phase II (Japan) | 2 years | 162 | TCZ (4 mg/kg, e.4.w) monotherapy TCZ (8 mg/kg, e.4.w) monotherapy PBO |
TCZ (8 mg/Kg) > TCZ (4 mg/Kg) | 31 | |
| CHARISMA Phase II (Europe) | MTX-IR | 16 weeks | 359 | TCZ (4 mg/kg, e.4.w) monotherapy TCZ (8 mg/kg, e.4.w) monotherapy TCZ (4 mg/kg, e.4.w) + MTX TCZ (8 mg/kg, e.4.w) + MTX PBO |
Well tolerated and efficacious TCZ (8 mg/Kg) > TCZ (4 mg/Kg) MTX combination > monotherapy |
32 |
| STREAM long term extension (Japan) | Phase I/II patients in Japan | 5 year extension | 143 | TCZ (8 mg/kg, e.4.w) monotherapy | Well tolerated and efficacious | 33 |
Abbreivations: MTX, methotrexate; TCZ, tocilizumab; IR, inadequate response; DMARDs, disease modifying antirheumatic drugs; PBO, placebo; wk, week; e.4.w, every 4 weeks.
The CHARISMA study (Chugai Humanized Anti-Rheumatic Interleukin Six Monoclonal Antibody) was performed in Europe.32 Active RA patients with an inadequate response (IR) to methotrexate (MTX) were randomly divided into one of the following seven treatment arms: TCZ at a dose of 2, 4 or 8 mg/kg that was administered either as monotherapy or in combination with MTX, or MTX plus a placebo. At week 16, the American College of Rheumatology (ACR) criteria for 20% improvement (ACR20) response was better in the 4 mg/kg and 8 mg/kg TCZ groups with or without MTX than in the group of patients that received the placebo plus MTX. Anti- TCZ antibodies were produced in approximately 25% of the TCZ 2 mg/kg monotherapy group.
To investigate the long-term safety and efficacy of TCZ, the STREAM study (the Long-term Safety and Efficacy of Tocilizumab, an Anti-IL-6 Receptor Monoclonal Antibody, in Patients with Rheumatoid Arthritis) was performed in Japan.33 Patients received TCZ monotherapy (8 mg/kg) that was administered every 4 weeks over a 5-year period. Overall, 32 patients (22%) withdrew from the study due to adverse events (AEs). After 5 years, 84.0, 69.1 and 43.6% of the patients met improvement criteria for ACR20, ACR50 and ACR70, respectively.
As shown in Table 2, seven phase III randomized clinical trials (RCTs) under various clinical situations have been conducted to evaluate the efficacy and safety of TCZ therapy of RA patients. In these studies, the TCZ dose was set as monthly administration at a dose of 4 or 8 mg/kg, based on the results of phase I and II studies.
Table 2.
Phase III randomized clinical trials of tocilizumab.
| Trial | Population studied | Administration duration | N | Treatment arm | ACR20 | DAS28 < 2.6 | Change of TSS | Ref. |
|---|---|---|---|---|---|---|---|---|
| AMBITION | MTX or Biologics without failure | 24 weeks | 286 | TCZ (8 mg/kg, e.4.w) + PBO | 70% | 34% | 34 | |
| 284 | MTX + PBO | 53% | 12% | |||||
| OPTION | MTX-IR | 24 weeks | 214 | TCZ (8 mg/kg, e.4.w) + MTX | 59% | 27% | 35,36 | |
| 205 | TCZ (4 mg/kg, e.4.w) + MTX | 48% | 13% | |||||
| 204 | PBO + MTX | 26% | 1% | |||||
| TOWARD | DMARDs-IR | 24 weeks | 803 | TCZ (8 mg/kg, e.4.w) + DMARDs | 61% | 30% | 37 | |
| 413 | PBO + DMARDs | 25% | 3% | |||||
| RADIATE | aTNF-IR | 24 weeks | 161 | TCZ (8 mg/kg, e.4.w) + MTX | 50% | 30% | 38 | |
| 170 | TCZ (4 mg/kg, e.4.w) + MTX | 30% | 8% | |||||
| 158 | PBO + MTX | 10% | 2% | |||||
| LITHE | MTX-IR | 24 weeks | 395 | TCZ (8 mg/kg, e.4.w) + MTX | 56% | 47% | 0.29 | 39 |
| 398 | TCZ (4 mg/kg, e.4.w) + MTX | 47% | 30% | 0.34 | ||||
| 393 | PBO + MTX | 25% | 8% | 1.13 | ||||
| SAMURAI | DMARDs-IR | 52 weeks | 157 | TCZ (8 mg/kg, e.4.w) | 78% | 59% | 2.3 | 40 |
| 145 | DMARDs | 34% | 3% | 6.1 | ||||
| SATORI | MTX-IR | 24 weeks | 61 | TCZ (8 mg/kg, e.4.w) + PBO | 80% | 43% | 41 | |
| 64 | MTX + PBO | 25% | 2% |
Abbreivations: MTX, methotrexate; TCZ, tocilizumab; IR, inadequate response; DMARDs, disease modifying antirheumatic drugs; aTNF, TNF inhibitor; PBO, placebo; ACR20, ACR criteria for 20% improvement; e.4.w, every 4 weeks; TSS, total sharp score.
The AMBITION trial (Actemra versus Methotrexate double-Blind Investigative Trial In mONotherapy) demonstrated that TCZ monotherapy is better than MTX monotherapy, showing rapid improvement in RA signs and symptoms, and a favorable benefit-risk, in patients for whom treatment with MTX or biological agents had not previously failed.34
The OPTION trial (Tocilizumab Pivotal Trial in Methotrexate Inadequate RespONders) demonstrated that TCZ in combination with MTX was effective and well tolerated in patients with moderate to severe active RA that had an inadequate response to MTX.35,36
The TOWARD trial (Tocilizumab in cOmbination With traditional DMARD therapy) demonstrated that TCZ combined with any of the disease modifying antirheumatic drugs (DMARDs) (MTX, chloroquine, gold, sulphasalazine, azathioprine or leflunomide) was safe and effective in reducing articular and systemic symptoms in patients with an inadequate response to these DMARDs.37
The RADIATE trial (RheumAtoiD ArthrItis study in Anti-TNF failurEs) demonstrated that TCZ plus MTX was effective in achieving rapid and sustained improvements in signs and symptoms in patients with an adequate response to TNF antagonists and had a manageable safety profile.38
The LITHE trial (TociLIzumab Safety and THE Prevention of Structural Joint Damage) demonstrated that TCZ plus MTX had superior ACR20 responses at 24 weeks compared with controls treated with placebo plus MTX and significantly inhibited radiographic progression at 52 weeks in patients with an inadequate clinical response to MTX.39
The SAMURAI (Study of Active Controlled Monotherapy Used for Rheumatoid Arthritis, an IL-6 inhibitor) trial demonstrated that TCZ monotherapy is generally well tolerated and provides radiographic benefit at 52 weeks in patients with an inadequate response to DMARDs.40
The SATORI trial (Study of Active Controlled TOcilizumab Monotherapy for Rheumatoid Arthritis Patients with an Inadequate Response to Methotrexate) demonstrated that TCZ monotherapy showed superior ACR response criteria compared with MTX plus placebo treatment for patients with an inadequate response to MTX.41
Phase IIIb/IV Clinical Studies
Following phase III clinical trials, a number of additional studies (TAMARA, DANBIO registry, REACTION, ROSE, ACT-RAY, ACT-SURE, and ACT-STAR) were conducted to investigate the efficacy of TCZ in clinical trials and daily clinical settings (Table 3).
Table 3.
Phase IIIb, IV and open-label clinical trials of tocilizumab.
| Study | Population studied | Design (country) | Administration duration | N | Treatment arm | Conclusion | Ref. |
|---|---|---|---|---|---|---|---|
| TAMARA (Phase IIIb) | DMARDs-IR | RCT (Germany) | 24 weeks | 286 | TCZ (8) monotherapy TCZ (8) + DMARDs |
TCZ was effective in daily clinical practice | 44,45 |
| DAMBIO (Phase IV) | DMARDs-IR or aTNF-IR | Retrospective cohort (Denmark) | 48 weeks | 178 | TCZ was effective in daily clinical practice | 46 | |
| REACTION | DMARDs-IR or aTNF-IR | Retrospective cohort (Japan) | 2 years | 229 | TCZ was effective in daily clinical practice | 47–49 | |
| ROSE (Phase IIIb) | DMARDs-IR | RCT (USA) | 24 weeks | 619 | TCZ (8) monotherapy PBO |
TCZ monotherapy was effective | 52 |
| Japanese PMS (Phase IV) | DMARDs-IR or aTNF-IR | Retrospective cohort (Japan) | 28 weeks | 7901 | TCZ was effective in daily clinical practice | 66,67 | |
| Post hoc analysis of clinical data | Patients of LITHE, OPTION, RADIATE study | Observational open-label (International) | Start at week 16 end at week 24 | 714 | TCZ (4) IR→TCZ (8) TCZ (8) IR→continues TCZ (8)→continues |
Dose escalation to 8 mg/kg was effective in TCZ 4 mg/kg IR | 43 |
| ACT-RAY (Phase IIIb) | MTX-IR | RCT (International) | 2 years | 556 | TCZ (8) monotherapy TCZ (8) + MTX |
TCZ monotherapy was not inferior to combined therapy with MTX | 53–55 |
| ACT-SURE (Phase IIIb) | DMARDs-IR or aTNF-IR | Observational open-label (International) | 6 months | 1681 | TCZ (8) monotherapy TCZ (8) + DMARDs |
TCZ monotherapy was not inferior to combined therapy with DMARDs | 56,57 |
| ACT-STAR | aTNF-IR | Observational open-label (USA) | 24 weeks | 886 | TCZ (4/8) + DMARDs TCZ (8) + DMARDs TCZ (8) monotherapy |
TCZ monotherapy was not inferior to combined therapy with DMARDs | 58,59 |
| DREAM | Remission after TCZ monotherapy | Retrospective cohort (Japan) | 12 weeks | 187 | Cessation of TCZ | Cessation of TCZ was possible | 61 |
| RESTORE | Recurrence after TCZ cessation | Retrospective cohort (Japan) | 12 weeks | 187 | Retreatment of TCZ | Retreatment with TCZ was acceptable | 62 |
Abbreviations: MTX, methotrexate; TCZ, tocilizumab; IR, inadequate response; DMARDs, disease modifying antirheumatic drugs; aTNF, TNF inhibitor; PBO, placebo; ACR20, ACR criteria for 20% improvement; TCZ (8), 8 mg/kg TCZ every 4 weeks; TCZ (4), 4 mg/kg TCZ every 4 weeks.
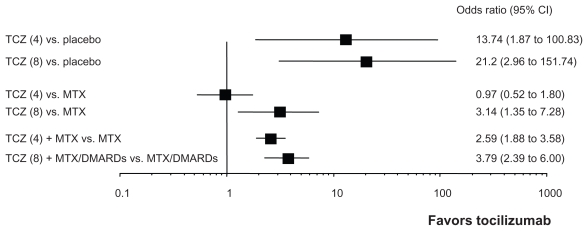
Compared with the EU and Japan, the recommended initial dosing of TCZ is low (4 mg/kg) in the USA. As shown in Figure 3, Cochrane review demonstrated that the efficacy of 4 mg/kg TCZ was inferior to that of 8 mg/kg TCZ under monotherapy condition.42 Therefore, dose-escalation of TCZ is often necessary in real life medical practice. A dose- escalation study in a clinical practice setting was therefore conducted in the US. The dose of TCZ for DMARDs inadequate response (IR) and TNF-IR patients who did not achieve adequate response to 4 mg/kg TCZ at 16 weeks was escalated to 8 mg/kg TCZ. These patients showed significant improvement when evaluated at 24 weeks.43
Figure 3.
The summary of the efficacy of tocilizumab in phase III randomized controlled trials in RA patients.
Notes: Cochrane review summarized the efficacy of tocilizumab (TCZ) from eight randomized controlled trials.42 These forest plots demonstrate odds ratio of achieving ACR 50 response rate. Efficacy of 8 mg/kg TCZ as monotherapy or in combination with MTX was significantly better than placebo or MTX in achieving ACR 50 response rates. Although the efficacy of 4 mg/kg TCZ monotherapy was not superior to MTX, that of 4 mg/kg with MTX can overcome MTX.
In Germany, 286 patients were registered for the TAMARA (Tocilizumab And DMARDs: Achievements in Rheumatoid Arthritis) study, which analyzed the effectiveness and safety of TCZ.44,45 Of these patients, 41.6% had been previously treated with TNF inhibitors. The percentage of patients that achieved DAS remission and a European League Against Rheumatism (EULAR) ‘good response’ was 47.6% and 54.9%, respectively. ACR50/70 response rates at week 24 were 50.7% and 33.9%, respectively. The new ACR/EULAR Boolean-based criteria were 15.0% after 12 weeks and 20.3% after 24 weeks. Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI) remission rates were 24.1% and 25.2%, respectively.
In Denmark, 178 patients with RA that were treated with TCZ were identified in the DANBIO (The Danish Registry for Biologic Therapies in Rheumatology) registry.46 After 48 weeks, 64% of these patients were still being treated. Among patients with available response data, DAS28 was 5.4, 2.9 and 2.5 at baseline, week 24 and week 48, respectively. The remission rates at weeks 24 and 48 were 39% and 58%, respectively and EULAR good or moderate response rates were 77% and 84%, respectively. These response rates were comparable to those found in patients switching to a second TNF inhibitor and to the response rates previously demonstrated in phase III clinical trials.
In Japan, 229 patients were registered in the REACTION (Retrospective Actemra Investigation for Optimal Needs of RA) study, which analyzed TCZ effectivity.47–49 DAS28 remission (DAS28 < 2.6) rates at 24, 52 and 104 weeks were 54%, 59% and 71%, respectively. CDAI remission (CDAI ≤ 2.8) rates at 24, 52, 104 weeks were 15%, 22%, and 37%, respectively. Structural remission (total sharp score ≤0.5) and functional remission (health assessment questionnaire ≤0.5) at 104 weeks were 53% and 38%, respectively. Thus, not only clinical remission but also structural and functional remission was maintained long-term.
These studies confirm both the short-term efficacy and the long-term tolerability of TCZ not only in RCTs but also in real life clinical care. In the DAMBIO registry, the 2-year drug survival of patients treated with TCZ and TNF inhibitors was 70% and 40%–60%, respectively.46,50 We also demonstrated drug continuation rate of TCZ.51 The median survival of patients treated with TCZ, infliximab (IFX), etanercept (ETN) and adalimumab (ADA) was 2.7, 1.7, 2.2 and 1.1 years, respectively. The survival rate of patients treated with TCZ was significantly higher than that of patients treated with TNF inhibitors (P < 0.001). These results indicate that survival following treatment with TCZ is superior to that following TNF inhibitor treatment.
In the USA, 619 patients were registered in the ROSE (The Rapid Onset and Systemic Efficacy) study for analysis of the efficacy and safety of TCZ.52 This study was a 24-week, randomized, double-blind, placebo-controlled, parallel-group, multicenter phase IIIb clinical trial. The percentage of ACR20 and ACR50 responders was significantly higher for TCZ-treated versus placebo-treated patients as early as week 4 and continued up to week 24. Compared to the placebo-treated patients, more patients in the TCZ group achieved ACR70 responses beginning at week 8 (P < 0.01). Safety findings were consistent with the known TCZ safety profile. Rapid improvement in clinical outcomes was demonstrated as early as week 1 as shown by DAS28 scores, patient measures and CRP levels.
The ACT-RAY study was a double-blind 2-year phase IIIb study.53–55 In this study, 556 patients who were on stable doses of oral weekly MTX were randomly divided into groups that were treated with either 8 mg/kg TCZ plus continued MTX (TCZ + MTX group) or were switched to 8 mg/kg TCZ (TCZ + placebo group). Treatment efficacy was evaluated at week 24. Five hundred and twelve patients (92%) completed the initial 24-week period. Of the TCZ + MTX group, 71.8%, 45.1% and 24.7% achieved ACR20, 50 and 70 responses, respectively and 40.4% achieved DAS remission. Of the TCZ + placebo group, 70.7%, 46.9% and 25.7% achieved ACR20, 50 and 70 response, respectively, and 34.8% achieved DAS remission. There were no differences in the ACR scores and DAS remission rates between the two groups. The onset of drug efficacy was rapid. Rates of AEs, serious AEs, and serious infections per 100 patient-years (PY) were 491, 21, and 6 for the TCZ + MTX group and 467, 18, and 6 for the TCZ group, respectively, with the most frequent AEs and serious AEs being infection. This study also analyzed X-ray and MRI changes after TCZ therapy. Structural analysis also indicated no difference between TCZ monotherapy and TCZ combined with MTX therapy.
The ACT-SURE study was a phase IIIb, open-label, single-arm, 6-month study.56,57 In this study, 1,681 patients with inadequate responses to DMARDs or TNF inhibitors were registered. Patients were randomly divided into groups that were treated with 8 mg/kg TCZ alone every 4 weeks (TCZ monotherapy group) or 8 mg/kg TCZ in combination with DMARDs (TCZ + DMARDs group) and were evaluated at 24 weeks. Of the TCZ monotherapy group, 43.5% and 23.8%, achieved ACR50 and 70 responses, respectively, and 57.9%, 18.6% and 21.3% achieved DAS, CDAI and SDAI remission, respectively. Of the TCZ + DMARDs group, 47.2% and 26.8% achieved ACR50 and 70 responses, respectively and 49.8%, 20.0% and 21.5% achieved DAS, CDAI and SDAI remission, respectively. Thus, TCZ as monotherapy showed the same efficacy as TCZ + DMARDs.
The ACT-STAR study was a 24-week, prospective, open-label study that was performed in the US. In this study,58,59 886 patients with moderate-to-severe active RA who had an inadequate response to current biologic or nonbiologic DMARDs were registered and divided into random groups that were treated with 4 mg/kg TCZ + DMARDs, 8 mg/kg TCZ + DMARDs or 8 mg/kg TCZ monotherapy. At week 8, patients treated with 4 mg/kg TCZ + DMARDs who did not achieve ACR20 had their TCZ dose increased to 8 mg/kg. For patients on 8 mg/kg TCZ + DMARDs, the dose could be decreased any time for safety reasons. Seven hundred and thirty one (82.5%) patients completed the study. Over half of the 4 mg/kg TCZ-treated patients were eventually switched to 8 mg/kg TCZ. Of the 4 mg/kg TCZ + DMARDs subgroup, 43.1%, 22.8% and 6.9% achieved ACR20, 50 and 70 responses, respectively at week 24 and 17.6% achieved DAS remission. In the 8 mg/kg TCZ + DMARDs subgroup, 48.9%, 22.6% and 8.1% achieved ACR20, 50 and 70 responses, respectively at week 24 and 22.8% achieved DAS remission. In the 8 mg/kg TCZ monotherapy subgroup, 47.3%, 20.9%, 8.1% achieved ACR20, 50 and 70 responses, respectively at week 24 and 15.8% achieved DAS remission. Safety profiles and efficacy were similar for the TCZ monotherapy and TCZ-combination with DMARDs groups.
Remission has recently become the current treatment goal for RA. An increased number of patients have achieved this goal in clinical trials.60 The duration of TCZ efficacy after cessation of TCZ treatment following DAS28 remission was demonstrated in the DREAM (Drug free Remission after cessation of Actemra Monotherapy) study.61 Efficacy following cessation of TCZ treatment was 35.1% and 13.4% at 24 and 52 weeks, respectively. Serum levels of IL-6 and MMP-3 are useful markers for identifying patients who may be able to discontinue TCZ without acute disease flare-up. In addition, the result that TCZ could induce remission again following recurrence after TCZ cessation was demonstrated by the RESTORE (Retreatment Efficacy and Safety to Tocilizumab in patients with rheumatoid arthritis at Recurrence) study.62
Safety
Cochrane review summarized the safety profile of TCZ from 8 RCTs (3,334 participants; 2,233 treated with TCZ and 1,101 controls).42 It is demonstrated that patients treated with TCZ are 1.2 times more likely to have any AE (absolute%; 74% vs. 65%) and 0.6 times less likely to be withdrawn from treatment for any reason (absolute%; 8.1% vs. 14.9%). Nishimoto et al also reported the safety profile of TCZ monotherapy including 6 initial RCTs and 5 long-term extension studies in Japan.63 For these studies, 601 patients with a total exposure of 2,188 PY were enrolled. The medial treatment duration was 3.8 years. The incidence of AEs, including abnormal laboratory test results, was calculated as 465.1/100 PY. Infection is the most common serious AE (6.22/100 PY). Furthermore, a systematic literature review to assess the risk of AEs for RA patients treated with TCZ reported that pooled odds ratios (ORs) indicated a statistically significant increased risk of AEs for patients treated with 8 mg/kg of TCZ plus methotrexate compared with controls (OR = 1.53; 95%CI = 1.26–1.86) in addition to a heightened risk of infection (OR = 1.30; 95%CI = 1.07–1.58). However, no increase in the incidence of malignancy or hepatitis was detected.
The safety profile of TCZ combination therapy was reported in 2011.64 This analysis included 5 core phase III RCT, 2 ongoing extension trials, 1 clinical pharmacology study and 2 patient populations; a control population (4,199) and a TCZ-treated population (4,009). Overall AE and serious AE rates were 278.2/100 PY and 14.4/100 PY, respectively. These events included serious infections (4.7/100 PY), opportunistic infections (0.23/100 PY), gastrointestinal perforations (0.28/100 PY), malignancy (1.1/100 PY), myocardial infarction (0.25/100 PY) and stroke (0.19/100 PY). Another report demonstrated that analysis of the pooled ORs revealed a statistically significant increased risk of AEs in the 8 mg/kg TCZ with MTX group compared with controls (OR = 1.53; 95%CI = 1.26–1.86). The risk of infection was significantly higher in the 8 mg/kg TCZ with MTX group compared with controls (OR = 1.30; 95%CI = 1.07–1.58). In contrast, no increased incidence of malignancy, tuberculosis reactivation or hepatitis was seen.65
The safety profile of TCZ in real life medicalcare in Japan was reported in 2011.66,67 In Japan, all patients treated with TCZ were registered in the all-patient postmarketing surveillance (JPMS-TCZ) program and were observed for 28 weeks. From April 2008 to August 2010, 12,799 patients were registered in JPMS-TCZ. The interim analysis of 3,881 patients has been published66 and the final analysis of the 7,901 patients of JPMS-TCZ was presented in ACR2011.67 The incidence of total AEs and serious AEs was 43.9% and 9.6%, respectively. Infections and infestation were the most frequent AEs (11.1%) and serious AEs (0.5%). The incidence of serious AEs was higher in the patients whose disease duration was ≥10 years. The DAS and Boolean remission rate was higher in patients whose disease duration was <2 years and the remission rate was higher in TNF inhibitor-naïve patients. Combining MTX with TCZ did not influence the remission rates. Surprisingly the serious AEs of TCZ monotherapy were slightly higher than those of TCZ and MTX combination therapy. The standard mortality ratio of TCZ (1.15; 95%CI = 1.12–2.46) was comparable with that of a standard Japanese patient cohort (between 1.46; 95%CI = 1.32–1.60 and 1.90; 95%CI: 1.75–2.07).68
Long-term safety was also analyzed in RCTs.69 Rates of serious AEs, serious infections, and cardiovascular events have remained stable with continued exposure to TCZ in long-term clinical trials. Infection was the most frequent serious AE. The most commonly reported infections in RCT were pneumonia (0.9/100PY) and skin or soft tissue infections (0.9/100PY). The rates of serious infections remained stable for 5 years indicating that continued administration of TCZ did not increase the risk of such infections.
In the JPMS-TCZ study, the rate of serious respiratory infections was 1.77/100 PY in the TCZ cohort and 0.53/100 PY in a standard Japanese patient cohort.70 The standardized incidence ratio (SIR) of serious respiratory infection in the TCZ cohort was 2.35 (95%CI = 1.66–3.24), standardized for age, sex and corticosteroid use. This risk was comparable with that associated with TNF inhibitors. As with other biologics, careful observation is necessary during administration of TCZ. In particular, TCZ has a strong down-regulatory effect on acute-phase reactants such as CRP. As a result, the consensus opinion issued a special caution that CRP cannot be used as a diagnostic indicator for early infections in patients treated with TCZ.71 White blood cell count and neutrophil counts are usually decreased, especially just after TCZ injection, but this decrease was not related to the incidence of infection.72
Tuberculosis and opportunistic infections have been observed in clinical trials of TCZ (0.3/100PY).70 However, half of these events were reported as non-serious infections. The reactivation of tuberculosis is a major concern during TNF inhibitor treatment.73 In a mouse model, anti-IL-6R Ab-treated mice survived for more than 200 days after TB challenge, whereas the anti-TNF-α Ab-treated mice all died between 120 and 181 days.74 In addition, interferon (IFN)-γ induction by the tuberculosis antigen was suppressed in the presence of TNF inhibitors (IFX and ETN) but not in the presence of TCZ.75 Although it seems likely that the incidence of reactivation of tuberculosis is lower during TCZ treatment, further detailed study will be needed to clarify this point. The consensus opinion recommended that patients should be screened for tuberculosis. Some cases of localized Herpes zoster infection occurred in clinical trials, but the relationship between TCZ administration and these infections was unclear. Patients with active hepatitis C or B were excluded from the clinical trials, so no data has been reported regarding TCZ and HBV or HCV infections.
Clinical trials and the above-mentioned combined-analysis showed that the rate of gastrointestinal perforation following TCZ treatment was 0.26/100 PY.69 In the JPMS-TCZ study, 7 gastrointestinal perforations were reported in 6 patients.66 In the worldwide Roche clinical trials, 26 (0.65%) cases of gastrointestinal perforation were found among patients with RA treated with TCZ at a rate of 1.9/1,000 PY and most cases appeared to be complications of diverticulitis.76 This rate lies between the reported rate of gastrointestinal perforations for corticosteroids (3.9/1,000 PY) and TNF inhibitors (1.3/1,000 PY) in the United Health Care database. The concomitant use of corticosteroids or non-steroid anti-inflammatory drugs with TCZ may increase this risk. The consensus opinion states that TCZ should be used in caution in patients with history of GI perforation, intestinal ulcers or diverticulitis.
Increases in mean fasting levels of plasma lipids such as total cholesterol, low-density lipoprotein, triglycerides and high-density lipoprotein, were seen in 20%–30% of patients treated with TCZ. The increase in lipid level resulting from TCZ treatment is perhaps mediated by an affect on lipoprotein receptor expression, since it has been recently shown that overproduced IL-6 decreases blood lipid levels via upregulation of the very-low-density lipoprotein (VLDL) receptor.77 The consensus opinion recommended that lipid levels be monitored 1–2 month after initiation of treatment and then every 6 months. In spite of the elevation of lipids, combined analysis of the data of clinical trials showed no apparent increase in cardiac events in a follow-up of up to 5 years. In the analysis, rates of myocardial infarction (0.3/100 PY) and stroke (0.2/100 PY) did not exceed the expected rates of RA patients.69 In contrast, oxidative stress was extremely low in patients with RA treated with TCZ.78 Moreover, hemoglobin (Hb) A1c levels and insulin sensitivity were improved by TCZ treatment.79,80
Combined-analysis showed that the overall ratio of malignancy occurrence during TCZ treatment was 1.2/100 PY.69 This ratio did not increase with the duration of TCZ treatment over a median duration of 3.6 years. Furthermore, the SIR for malignancy was 1.1/100 PY, which was considered to be within the range expected of RA patients. Although there is no report regarding increased incidences of malignancy during TCZ therapy, the use of TCZ for treatment of RA patients with malignancy cannot be recommended.
Many patients treated with TCZ have shown a decrease in absolute neutrophil count. This decrease usually occurs early after administration and is transient. There was no clear relationship between this neutropenia and an increase in infections.72 The consensus recommended that complete blood counts should be monitored regularly (every 4–8 weeks).
Hepatic aminotransferase such as L-aspartate aminotransferase (AST) and L-alanine aminotransferase (ALT) and bilirubin elevations may occur in patients treated with TCZ. In the JPMS-TCZ study, abnormal hepatic function was observed in 8.5% and 5.5% of TCZ-treated patients with or without MTX.66,67 Bilirubin elevations, mostly indirect, may occur independently. Liver function should be monitored regularly.
Serious infusion reactions related to TCZ treatment are rare. In the combined analysis, 8 out of a total of 4,009 patients showed anaphylactic infusion reactions.69 Most of infusion reaction of TCZ occurs the beginning of TCZ therapy.
The safety of surgical intervention in patients with RA treated with TCZ has been reported.81 Postoperative surgical site infections were not increased during TCZ therapy. TCZ is recommended to be withheld at least 14 days before major surgical procedures.69
There are no data available regarding the safety of TCZ treatment during pregnancy and lactation. Although IL-6-deficient mice are perfectly viable,82 IL-6 was reported to reduce recurrent abortion in an animal model.83 However, the effect of TCZ on embryonic and fetal development is unknown. At present the use of TCZ throughout pregnancy cannot be recommended.
Place in Therapy
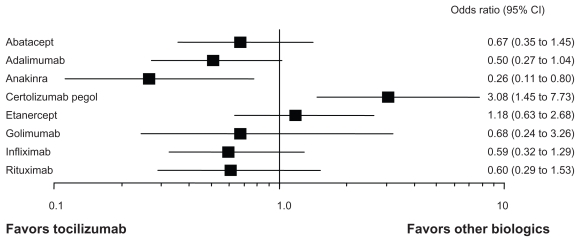
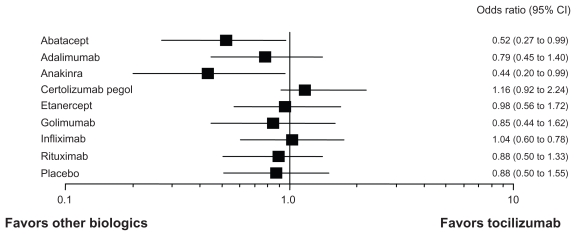
A number of biologics are currently available for the treatment of RA, including TNF inhibitors (infliximab; IFX, etanercept; ETN, adalimumab; ADA, golimumab; GOL, and certolizumab pegol; CEL), an IL-1 antagonist (anakinra; ANK), a B-cell depletor (rituximab; RTX), an IL-6 receptor inhibitor (TCZ), and a T-cell activation blocker (abatacept; ABA). While all of these biologics have proven effectiveness in RCTs, very limited head-to-head comparative studies are available. Recently, meta-analysis has allowed indirect comparisons between TCZ and other biologics. Bergman et al reported such an indirect comparison of TCZ and other biologics.84 The effectiveness of TCZ appeared to be comparable to that of other biologics (ABA, ADA, ETN, IFX and RTX) in terms of ACR20 and ACR50 responses. Turkstra et al reported a mixed treatment comparison of the short-term efficacy of nine biologics (ABA, ADA, ANK, CEL, ETN, GOL, IFX, RTX, and TCZ) in patients with established RA.85 As shown in Figure 4, the ACR50 response rate of TCZ at 6 months is comparable to that of ABA, ADA, ETN, GOL, IFX and RTX. ANK is inferior to TCZ and CEL may be superior to TCZ. Salliot et al reported an indirect comparison of the efficacy of TCZ in RA patients with inadequate response to TNF inhibitors (ADA, ETN and IFX).86 No significant difference was found between TCZ and other biologics (ABA, GOL, and RTX). A comparison of AEs of nine biologics was reported in Cochrane review.87 As shown in Figure 5, serious AEs of TCZ were comparable to those of other biologics except for ABA and ANK. These indicate that the efficacy and tolerability of TCZ is comparable to TNF inhibitors. Although TNF inhibitors are now recommended as first-line biologics,88 TCZ also has a potential capacity as a first-line biologic for RA patients who cannot use sufficient MTX, or is appropriate for the treatment of RA patients with secondary failure of TNF inhibitors. In the real world of rheumatology practice, there are RA patients who cannot use sufficient MTX because of AEs. TEMPO89 and PREMIER90 studies demonstrated no substantial differences in clinical responses between TNF inhibitors used as monotherapy (ETN and ADA) and MTX.91 British Society for Rheumatology Biologics Register (BSRBR) also concluded better responses of TNF inhibitors, when used in combination with MTX, than those used as monotherapy.92 In contrast, ACT-RAY, ACT-SURE and ACT-STAR indicated that there was no difference of the efficacy between TCZ monotherapy and that with MTX.53–59 These results suggest that TCZ has advantage for the treatment of RA patients who are not tolerable to receive MTX, since TCZ provides the excellent efficacy even without concomitant use of MTX.
Figure 4.
Indirect comparison of the efficacy of toclizumab and other biologics in RA.
Notes: Indirect comparison of ACR50 response rate at 6 months-treatment of biologics (tocilizumab vs. 8 other biologics).85 The efficacy of tocilizumab is comparable to other biologics except for certolizumab pegol and anakinra. Other analyses of the efficacy (tocilizumab vs. TNF inhibitors) show similar results.84,86
Figure 5.
Indirect comparison of serious adverse events of tocilizumab and other biologics in RA.
Notes: Indirect comparison of serious adverse events of biologics (tocilizumab vs. 8 other biologics and placebo).87 The safety profile of tocilizumab is comparable to other biologics except abatacept and anakinra.
In the real world of rheumatology practice, there are also RA patients who fail to respond to TNF inhibitors (primary failure) or lose response to them (second failure).93 In such case, the switching of biologics is required. However, the probability of responding to a second TNF inhibitor is lower than that of responding to the first one.94 For patients with primary failure it is conceivable that TNF may not be a major effector cytokine, so that change of target from TNF to non-TNF molecules including IL-6 is reasonable. TCZ is also efficacious for patients with second TNF failure.38,44,45,56–59 These findings indicate TCZ is a good indication for the treatment of RA patients who are intolerable to MTX or non-responders to TNF inhibitors. Finally, it is worthy of describing here that clinical trial of subcutaneous injection of TCZ is currently undergoing. If the subcutaneous TCZ injection will bring the similar efficacy to the intravenous one, it would be convenient for patients.
Conclusion
IL-6 is a key cytokine in immune activation and inflammation. TCZ exerts a blocking effect on IL-6 function and exhibits excellent efficacy and tolerability for RA treatment. Because of limited evidence of TCZ in the USA, at the present time the ACR recommendations for the treatment of RA does not recommend TCZ as a first-line biologic. However, recent advance indicates that TCZ has a certain advantage for patients who cannot use MTX or fail to respond to TNF inhibitors. We believe that TCZ possesses a capacity as a first-line biologic for the treatment of RA patients, although further clinical experience of TCZ will be required.
Footnotes
Conflict of Interest
A.O. has received a consulting fee, as a medical adviser from Chugai Pharmaceutical Co., Ltd. The other authors declare no conflict of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–6. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 3.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto T. Interleukin-6: from basic science to medicine-40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 7.Nishimoto N, Kishimoto T. Interleukin-6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–26. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol. 2010;6:843–54. doi: 10.1586/eci.10.70. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Narazaki M, Kishimoto T. Therapeutic targeting of the interleukin-6 receptor. Annu Rev Pharmacol Toxicol. 2012;52:199–219. doi: 10.1146/annurev-pharmtox-010611-134715. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–74. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 11.Kotake S, Sato K, Kim KJ, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 12.Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activation of NF-kappa B in mouse calvariae. J Immunol. 2002;169:3353–62. doi: 10.4049/jimmunol.169.6.3353. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47:1635–40. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 14.Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–9. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 15.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–8. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 16.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–4. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;16;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takai Y, Seki N, Senoh H, et al. Enhanced production of interleukin-6 in mice with type II collagen-induced arthritis. Arthritis Rheum. 1989;32:594–600. doi: 10.1002/anr.1780320513. [DOI] [PubMed] [Google Scholar]
- 19.Takagi N, Mihara M, Moriya Y, et al. Blockade of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 1998;41:2117–21. doi: 10.1002/1529-0131(199812)41:12<2117::AID-ART6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Sasai M, Saeki Y, Ohshima S, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Mihara M, Kotoh M, Nishimoto N, et al. Humanized antibody to human interleukin-6 receptor inhibits the development of collagen arthritis in cynomolgus monkeys. Clin Immunol. 200;98:319–26. doi: 10.1006/clim.2000.4989. [DOI] [PubMed] [Google Scholar]
- 22.Uchiyama Y, Yorozu K, Hashizume M, Moriya Y, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, ameliorates joint swelling in established monkey collagen-induced arthritis. Biol Pharm Bull. 2008;31:1159–63. doi: 10.1248/bpb.31.1159. [DOI] [PubMed] [Google Scholar]
- 23.Kagari T, Doi H, Shimozato T. The importance of IL-1 beta and TNF-alpha, and the noninvolvement of IL-6, in the development of monoclonal antibody- induced arthritis. J Immunol. 2002;169:1459–66. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 24.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17 A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogata A, Umegaki N, Katayama I, Kumanogoh A, Tanaka T. Psoriatic arthritis in two patients with an inadequate response to treatment with tocilizumab. Joint Bone Spine. 2011 September 29; doi: 10.1016/j.jbspin.2011.06.011. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 26.Patel AM, Moreland LW. Interleukin-6 inhibition for treatment of rheumatoid arthritis: a review of tocilizumab therapy. Drug Des Devel Ther. 2010;4:263–78. doi: 10.2147/DDDT.S14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narazaki M, Kawai M, Morishima Y, et al. Insufficient effects of tocilizumab on the patients with rheumatoid arthritis who have splenomegaly. Presented at EULAR 2010 Rome; abstract SAT0272. [Google Scholar]
- 28.Nishimoto N, Terao K, Mima T, Nakahara H, Takagi N, Kakehi T. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood. 2008;112:3959–64. doi: 10.1182/blood-2008-05-155846. [DOI] [PubMed] [Google Scholar]
- 29.Choy EH, Isenberg DA, Garrood T, et al. Therapeutic benefit of blocking interleukin-6 activity with an antiinterleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46:143–50. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto N, Yoshizaki K, Maeda K, et al. Toxicity, pharmacokinetics, and dose finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol. 2003;30:1426–35. [PubMed] [Google Scholar]
- 31.Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–9. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 32.Maini RN, Taylor PC, Szechinski J, et al. CHARISMA Study Group. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 33.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-IL-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580–4. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. OPTION Investigators. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial. Lancet. 2008;371:987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 36.Garnero P, Thompson E, Woodworth T, Smolen JS. Rapid and sustained improvement in bone and cartilage turnover markers with anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate: results from a substudy of the multicenter double-blind, placebo controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 2010;62:33–43. doi: 10.1002/art.25053. [DOI] [PubMed] [Google Scholar]
- 37.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with TCZ reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the TCZ in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–80. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 38.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with TCZ improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-TNF biologics: results from a 24-week multicentre randomized placebo controlled trial. Ann Rheum Dis. 2008;67:1516–23. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–21. doi: 10.1002/art.30158. [DOI] [PubMed] [Google Scholar]
- 40.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x-ray reader-blinded randomized controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–67. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishimoto N, Miyasaka N, Yamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–9. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh JA, Beg S, Lopez-Olivo MA. Tocilizumab for rheumatoid arthritis. Cochrane Database Syst Rev. 2010 Jul 7;70:CD008331. doi: 10.1002/14651858.CD008331.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Curtis JR, Ogale S, Devenport J, Lepley D. Effects of tocilizumab dose escalation on disease activity in adult rheumatoid arthritis patients with inadequate response at 16 weeks. Presented at ACR 2011; Cicago. Abstract 2219. [Google Scholar]
- 44.Burmester GR, Feist E, Kellner H, Braun J, Iking-Konert C, Rubbert-Roth A. Effectiveness and safety of the interleukin 6-receptor antagonist tocilizumab after 4 and 24 weeks in patients with active rheumatoid arthritis: the first phase IIIb real-life study (TAMARA) Ann Rheum Dis. 2011;70:755–9. doi: 10.1136/ard.2010.139725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iking-Konert C, Aringer M, Wollenhaupt J, et al. Performance of the new 2011 ACR/EULAR remission criteria with tocilizumab using the phase IIIb study TAMARA as an example and their comparison with traditional remission criteria. Ann Rheum Dis. 2011;70:1986–90. doi: 10.1136/ard.2011.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leffers HC, Ostergaard M, Glintborg B, et al. all departments of rheumatology in Denmark. Efficacy of abatacept and tocilizumab in patients with rheumatoid arthritis treated in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis. 2011;70:1216–22. doi: 10.1136/ard.2010.140129. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka H, Tanaka Y, Inoue E, et al. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: results from a retrospective study (REACTION study) Mod Rheumatol. 2011;21:122–33. doi: 10.1007/s10165-010-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi T, Tanaka Y, Amano K, et al. Clinical, radiographic and functional effectiveness of tocilizumab for rheumatoid arthritis patients-REACTION 52-week study. Rheumatology (Oxford) 2011;50:1908–15. doi: 10.1093/rheumatology/ker221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanaka Y, Takeuchi T, Amano K, et al. Impact of tocilizumab therapy for remission quartet in rheumatoid arthritis—the result of 104 weeks follow up data of REACTION study. Presented at ACR 2011; Cicago. Abstract 1237. [Google Scholar]
- 50.Hetland ML, Christensen IJ, Tarp U, et al. All Departments of Rheumatology in Denmark. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22–32. doi: 10.1002/art.27227. [DOI] [PubMed] [Google Scholar]
- 51.Hishitani Y, Hirano T, Shima Y, et al. Long-term tolerability of tocilizumab for the treatment of rheumatoid arthritis. Presented at ACR 2011; Chicago. Abstract 2243. [Google Scholar]
- 52.Yazici Y, Curtis JR, Ince A, et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann Rheum Dis. 2011 September 26; doi: 10.1136/ard.2010.148700. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 53.Troum O, Peterfy C, Olech E, et al. Early reductions in synovitis and osteitis with tocilizumab therapy are maintained through week 54: Results from the ACT-RAY MRI substudy. Presented at EULAR 2011; London. Abstract SAT0282. [Google Scholar]
- 54.Dougados M, Huizinga T, Sheeran TT, et al. Tocilizumab (TCZ) plus methotrexate (MTX) does not have superior clinical efficacy to TCZ alone in RA patients with inadequate response to MTX: 24-week results of the ACT-RAY study. Presented at EULAR 2011; London. Abstract OP0020. [Google Scholar]
- 55.Dougados M, Kissel K, Amital H, et al. Double-blind study of tocilizumab plus methotrexate vs tocilizumab plus placebo in patients with active rheumatoid arthritis despite prior methotrexate: progression of structural damage, quality of life, and physical function at 24 weeks. Presented at ACR 2011; Cicago. Abstract 2628. [Google Scholar]
- 56.Bykerk V, Alvaro-Gracia J, Román Ivorra J, et al. ACT-SURE Study Group. Tocilizumab treatment in patients with rheumatoid arthritis and inadequate response to DMARDs and/or the inhibitors: ACT-SURE final results. Presented at EULAR 2011; London. Abstract SAT0300. [Google Scholar]
- 57.Bykerk V, Östör A, Ivorra JAR, et al. ACT-SURE Study Group. A comparison of tocilizumab as monotherapy or with add-on disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and an inadequate response to previous treatments. Presented at ACR 2011; Cicago. Abstract 2218. [Google Scholar]
- 58.Weinblatt M, Kremer J, Cush J, et al. Safety of tocilizumab (TCZ) and TCZ plus DMARDs in US RA population with inadequate response (IR) to biologics or DMARDs: The ACT-STAR study. Presented at EULAR 2011; London. Abstract LB0006. [Google Scholar]
- 59.Weinblatt M, Kremer J, Cush J, et al. Tocilizumab monotherapy and tocilizumab plus disease-modifying antirheumatic drugs in a US rheumatoid arthritis population with inadequate response to anti-tumor necrosis factor agents. Presented at ACR 2011; Cicago. Abstract 427. [Google Scholar]
- 60.Van den Broek M, Huizinga TW, Dijkmans BA, Allaart CF. Drug-free remission: is it already possible? Crurr Opin Rheumatol. 2011;23:266–72. doi: 10.1097/BOR.0b013e32834563e3. [DOI] [PubMed] [Google Scholar]
- 61.Nishimoto N Japanese MRA study group for RA. Drug free remission after cessation of actemra monotherapy (DREAM Study). Presented at EULAR 2010; Roma. Abstract OP0134. [Google Scholar]
- 62.Nishimoto N Japanese MRA study group for RA. Retreatment efficacy and safety to Tocilizumab in patients with rheumatoid arthritis at recurrence (RESTORE study). Presented at EULAR 2010; Roma. Abstract SAT0150. [DOI] [PubMed] [Google Scholar]
- 63.Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222–32. doi: 10.1007/s10165-010-0279-5. [DOI] [PubMed] [Google Scholar]
- 64.Schiff MH, Kremer JM, Jahreis A, Vernon E, Isaacs JD, van Vollenhoven RF. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13:R141. doi: 10.1186/ar3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell L, Chen C, Bhagat SS, Parker RA, Ostor AJ. Risk of adverse events including serious infections in rheumatoid arthritis patients treated with tocilizumab: a systematic literature review and meta-analysis of randomized controlled trials. Rheumatology (Oxford) 2011;50:552–62. doi: 10.1093/rheumatology/keq343. [DOI] [PubMed] [Google Scholar]
- 66.Koike T, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan: interim analysis of 3881 patients. Ann Rheum Dis. 2011;70:2148–51. doi: 10.1136/ard.2011.151092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamanaka H, Harigai M, Inokuma S, et al. Postmarketing surveillance of tocilizumab for rheumatoid arthritis in Japan-full analysis report of 7,901 patients. Presented at ACR 2011; Cicago. Abstract 2629. [Google Scholar]
- 68.Nakajima A, Inoue E, Tanaka E, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol. 2010;39:360–7. doi: 10.3109/03009741003604542. [DOI] [PubMed] [Google Scholar]
- 69.Genovese MC, Sebba A, Rubbert-Roth A, et al. Long-term safety of tocilizumab in rheumatoid arthritis clinical trials. Presented at ACR 2011; Cicago. Abstract 2217. [Google Scholar]
- 70.Hoshi D, Nakajima A, Inoue E, et al. Incidence of serious respiratory infections in patients with rheumatoid arthritis treated with tocilizumab. Mod Rheumatol. 2011 July 8; doi: 10.1007/s10165-011-0488-6. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 71.Furst DE, Keystone EC, Braun J, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2010. Ann Rheum Dis. 2011 Mar;70(Suppl 1):i2–36. doi: 10.1136/ard.2010.146852. [DOI] [PubMed] [Google Scholar]
- 72.Smolen JS, Van Vollenhoven R, Rubbert-Roth A, et al. Analysis of baseline data and neutrophil counts in patients with serious infections from two tocilizumab clinical trials. Presented at EULAR 2008; Paris. Abstract THU0169. [Google Scholar]
- 73.Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122–7. doi: 10.1002/art.11137. [DOI] [PubMed] [Google Scholar]
- 74.Okada M, Kita Y, Kanamaru N, et al. Anti-IL-6 receptor antibody causes less promotion of tuberculosis infection than anti-TNF-α antibody in mice. Clin Dev Immunol. 2011:404929. doi: 10.1155/2011/404929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ogata A, Mori M, Hashimoto S, et al. Minimal influence of tocilizumab on IFN-gamma synthesis by tuberculosis antigens. Mod Rheumatol. 2010;20:130–3. doi: 10.1007/s10165-009-0243-4. [DOI] [PubMed] [Google Scholar]
- 76.Gout T, Ostör AJ, Nisar MK. Lower gastrointestinal perforation in rheumatoid arthritis patients treated with conventional DMARDs or tocilizumab: a systematic literature review. Clin Rheumatol. 2011;30:1471–4. doi: 10.1007/s10067-011-1827-x. [DOI] [PubMed] [Google Scholar]
- 77.Hashizume M, Yoshida H, Koike N, Suzuki M, Mihara M. Overproduced IL-6 decreases blood lipid levels via upregulation of very-low-density lipoprotein receptor. Ann Rheum Dis. 2010;69:741–6. doi: 10.1136/ard.2008.104844. [DOI] [PubMed] [Google Scholar]
- 78.Hirao M, Yamasaki N, Oze H, et al. Serum level of oxidative stress marker is dramatically low in patients with rheumatoid arthritis treated with tocilizumab. Rheumatol Int. 2011 September 11; doi: 10.1007/s00296-011-2135-0. [Epub ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogata A, Morishima A, Hirano T, et al. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70:1164–5. doi: 10.1136/ard.2010.132845. [DOI] [PubMed] [Google Scholar]
- 80.Schultz O, Oberhauser F, Saech J, et al. Effects of inhibition of interleukin- 6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirao M, Hashimoto J, Tsuboi H, et al. Laboratory and febrile features after joint surgery in patients with rheumatoid arthritis treated with tocilizumab. Ann Rheum Dis. 2009;68:654–7. doi: 10.1136/ard.2008.090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poli V, Balena R, Fattori E, et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 1994;13:1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dubinsky V, Junovich G, Gentile T, Gutiérrez G. IL-6 as a regulatory factor of the humoral response during pregnancy. Am J Reprod Immunol. 2008;60:197–203. doi: 10.1111/j.1600-0897.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 84.Bergman GJ, Hochberg MC, Boers M, Wintfeld N, Kielhorn A, Jansen JP. Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin Arthritis Rheum. 2010;39:425–41. doi: 10.1016/j.semarthrit.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Turkstra E, Ng SK, Scuffham PA. A mixed treatment comparison of the short-term efficacy of biologic disease modifying anti-rheumatic drugs in established rheumatoid arthritis. Curr Med Res Opin. 2011;27:1885–97. doi: 10.1185/03007995.2011.608655. [DOI] [PubMed] [Google Scholar]
- 86.Salliot C, Finckh A, Katchamart W, et al. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis. 2011;70:266–71. doi: 10.1136/ard.2010.132134. [DOI] [PubMed] [Google Scholar]
- 87.Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum (Arthritis Care Res) 2008;59:762–84. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 89.Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–81. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 90.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 91.Donahue KE, Gartlehner G, Jonas DE, et al. Systematic review: comparative effectiveness and harms of disease-modifying medications for rheumatoid arthritis. Ann Intern Med. 2008;148:124–34. doi: 10.7326/0003-4819-148-2-200801150-00192. [DOI] [PubMed] [Google Scholar]
- 92.Hyrich KL, Symmons DP, Watson KD, et al. Comparison of the response to infliximab or etanercept monotherapy with the response to cotherapy with methotrexate or another disease-modifying antirheumatic drug in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:1786–94. doi: 10.1002/art.21830. [DOI] [PubMed] [Google Scholar]
- 93.Atzeni F, Sarzi-Puttini P, Gorla R, Marchesoni A, Caporali R. Switching rheumatoid arthritis treatments: an update. Autoimmun Rev. 2011;10:397–403. doi: 10.1016/j.autrev.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Gomez-Reino JJ, Carmona L BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients. Arthritis Res Ther. 2006;8:R29. doi: 10.1186/ar1881. [DOI] [PMC free article] [PubMed] [Google Scholar]