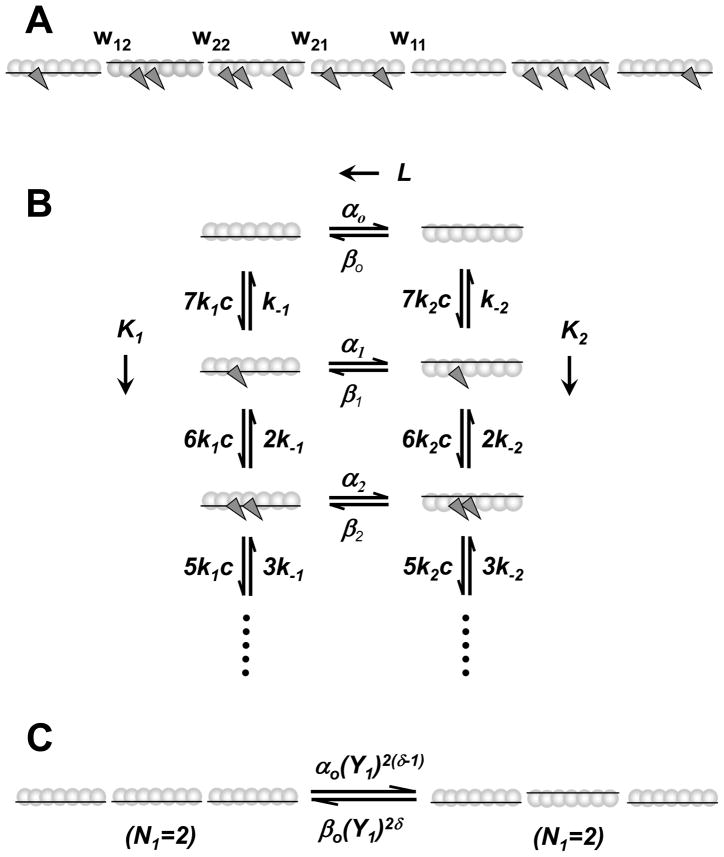
Figure 1.
The Hill two-state model schema. (A) A regulated actin filament is structurally composed as sequential repeat of TmTn complex each interacting with seven actin sites. The TmTn complex is denoted as horizontal line and actin monomers on a single F-actin strand are denoted as circles. Each TmTn complex can be in the inactive state (TmTn line down) and in the active state (TmTn line up). The equilibrium between these states in the absence of S1 in solution is solely defined by the Ca2+ concentration. In the presence of S1, the rate of myosin binding to regulated F-actin is regulated by the ratio of the inactive and active states. The binding of S1 (dark gray triangles) is permitted in both inactive and active actin7·TmTn states but it is severely inhibited in inactive state. The nearest-neighbor interactions between two neighboring TmTn units is defined by the interaction energy, wij, between i and j actin7·TmTn states. (B) The kinetic scheme showing the transitions between for actin7·TmTn states (columns), defined by intrinsic equilibrium constant, L = αo/βo, and the kinetics of myosin binding to either inactivated and activated states, defined by equilibrium constants, K1 = k1/k−1 and K2 = k2/k−2 respectively. Here c is the concentration of free S1 in solution, k1 and k2 are S1 binding rate constants, and k−1 and k−2 are rates of unbinding of S1 from F-actin. The transition rate constants of an actin7·TmTn unit between inactive and active states, αm and βm, depends on the number of S1 bound to the unit, m. (C) The cooperativity of TmTn units between inactive and active states depends on the number of the two neighbors that are in State 1, denoted as N1, the interaction energies Y1 = Y11Y12, and δ is a fixed constant between 0 and 1. Note that N1 can have values of 0, 1, or 2. The backward rate constants and other fixed parameters used in all simulations, unless are denoted in the figure legends differently, are taken to be βo = 300 s−1, Y2 = 0.208, k−1 = 500 s−1 and k−2 =0.09 s−1, γ =0.8 and δ =0.5.