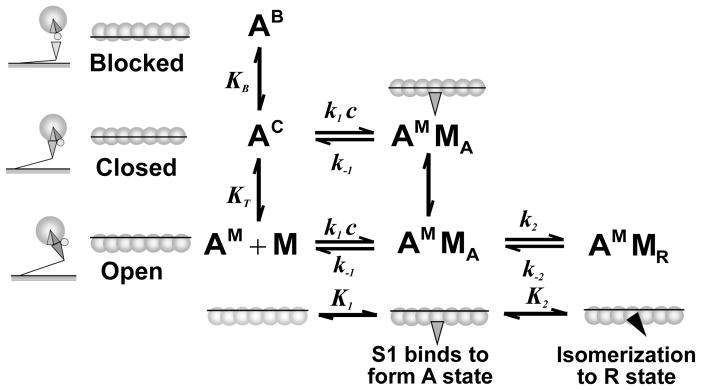
Figure 2.
Mckillop-Geeves (McK-G) three-state model scheme.8 In the McK-G model the structural unit, actin7·TmTn, is schematically shown as seven open circles representing the actin monomers connected via a line representing the tropomyosin. This unit exists in a dynamic equilibrium between the three states as represented by the different positions of tropomyosin: the blocked state, AB, in which no myosin-S1 binding can occur, the closed or calcium induced state, AC, in which only weak binding of S1 can occur, and the open or myosin induced state, AM, which allows isomerization of the myosin-S1 to the rigor-like state. The ratio of the three states in the absence of myosin-S1 is defined by the equilibrium constants KB (between the closed and blocked states) and KT (between the closed and open states). The blocked state is only present in the absence of Ca2+ when the Tn complex is tightly bound to the thin filament; in the presence of Ca2+, there is little or no occupancy of the blocked state. Weakly bound myosin states are denoted as A-states (AMMA) and rigor-like states are denoted as R-states (AMMR). The rate of myosin binding is defined by equilibrium constant K1= k1c/k−1, and the rate of isomeration of S1 into R state is defined by equilibrium constant K2 = k2/k−2. Backward rate constants used in all simulations are taken to be k−B = 100 s−1, k−T = 3000 s−1, k−1 = 10 s−1 and k−2 =5 s−1. Also the equilibrium constants were fixed over all simulations, unless is denoted differently in the figure legends are: K1 = 0.22 μM−1 and K2 = 200.