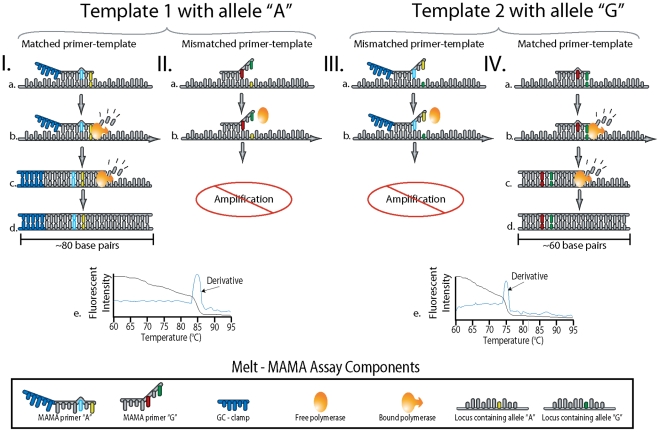
Figure 2. The principle of the Melt-MAMA PCR reaction.
Four different scenarios involving two alternate SNP allele templates (I & II vs. III & IV) and the interaction of Allele-Specific (AS) PCR amplification using MAMA primers. The annealing of AS-MAMA primers to their allelic templates is shown with one primer labeled with a 5′ GC-clamp (Ia) whereas the other is not (IVa). (Ib and IVb) Taq Polymerase extends from the 3′ matched AS-MAMA primer despite the antepenultimate destabilizing nucleotide. (Ic and IVc) The second PCR cycle replicates from a newly synthesized DNA template made in the previous step (Ib and IVb). With the synthesized DNA serving as the template, a perfect primer-template complex is formed eliminating the antepenultimate destabilizing mismatch observed in Iab and IVab. At PCR endpoint (Id and IVd), the amplicons generated from the 3′ matched AS-MAMA primer greatly outnumbers the amplicons generated by the mismatched AS-MAMA primer. Temperature-dissociation curve plots (Ie and IVe) of each AS-PCR product (Iabcd, IIab and IIIab, IVabcd), showing the fluorescent intensity and the rate of fluorescent intensity change (derivative) as a function of temperature. For each allelic template reaction (I & II vs. III & IV), the melt profiles (Ie and IVe) show only a single change in fluorescent intensity. This indicates the amplification of the perfect-matched amplicon and little to no amplification of the mismatched amplicon. The GC –clamp “labeled” amplicons dissociate at higher temperatures (∼3°C to 5°C) than non-GC amplicons. Nonproductive primer annealing is shown for an AS-MAMA primer (IIa) and a GC-clamp AS-MAMA primer (IIIa) binding with their respective corresponding mismatched templates. The lack of Watson-Crick base pairing at two 3′ positions (the antepenultimate nucleotide at the 3′ end) of the AS primer introduces instability at this region (IIb and IIIb). This prevents efficient extension by the polymerase, which retards or prevents product amplification (Ie and IVe).