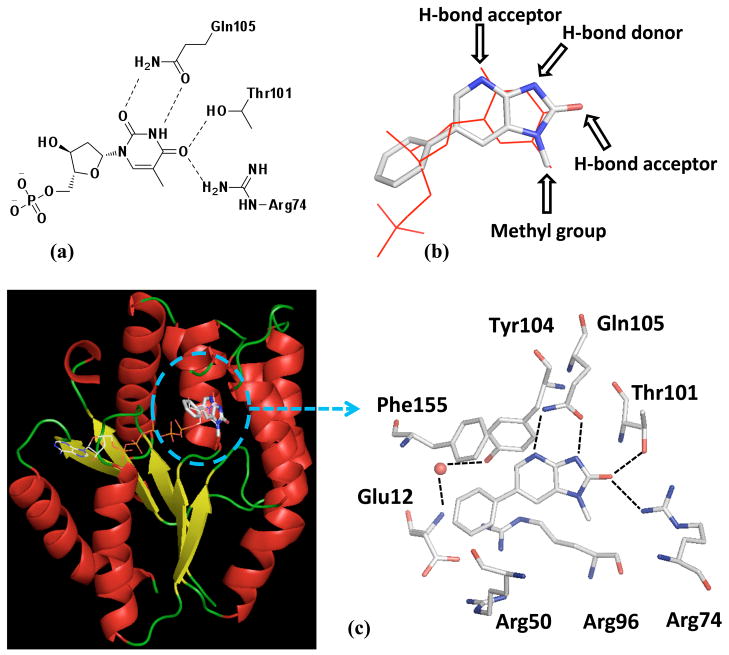
Figure 2.
Key interactions of dTMP and 1 in PaTMK. (a) Thymidine interactions in the active site of PaTMK at the homology model are represented in two-dimensional view; (b) The result of flexible alignment of 1 to dTMP shows clear pharmacophore match between 1 and dTMP. The structure of dTMP is represented as red line, and that of 1 is as stick with atom-type color (gray for carbon, blue for nitrogen, red for oxygen, and orange for phosphorous); (c) The overall X-ray co-crystal structure of PaTMK and 1 shows 1 binds where dTMP bound (left panel). The structure of TP5A was merged from the reported X-ray structure (PDB ID: 4TMK) to compare similarity of binding site of 1 and TP5A. The binding mode of 1 in PaTMK at the X-ray co-crystal structure shows the binding pose of 1 at PaTMK is identical to that of dTMP. α-Helix is red, β-sheet is yellow, and loop is green (left panel). The water molecule is represented with a red sphere, 1 and amino acids associated with the binding of 1 are represented as sticks with atom-type color, and H-bond interactions are represented with black lines. π-Cation stacking between the phenyl ring of 1 and Arg96 is not marked. Hydrogen atoms are omitted for clarity. Figures are generated with Pymol program.