Fig. 3.
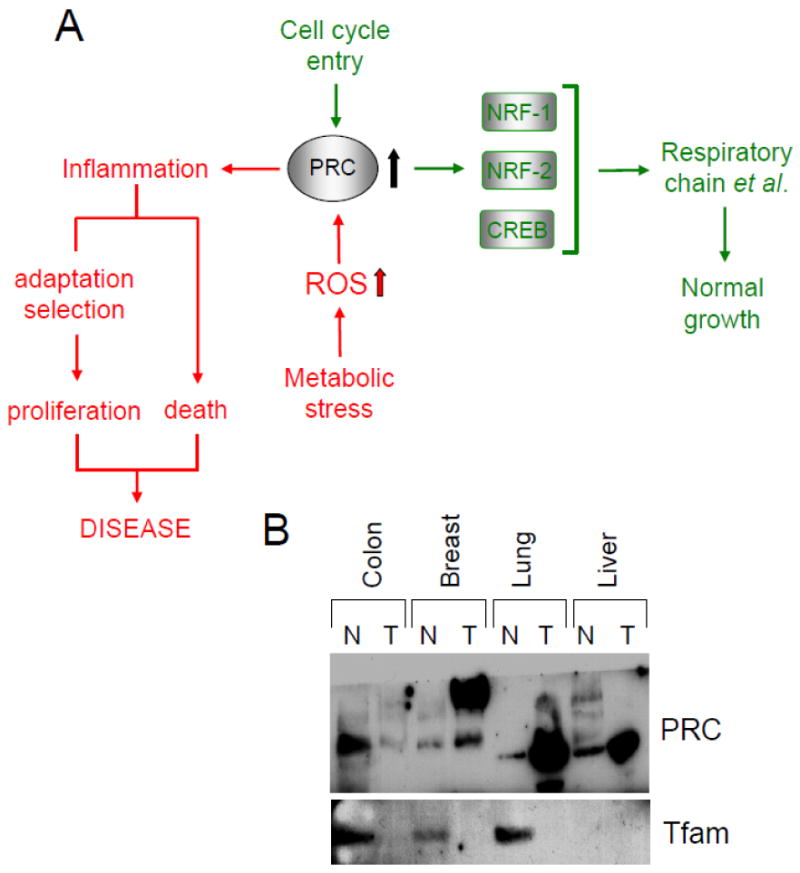
A potential role for PRC in orchestrating a balance between cell cycle entry and the inflammatory response to metabolic stress. (Panel A) Under normal conditions (green pathway), PRC protein levels are transiently upregulated during entry to the cell cycle. This coincides with the normal initiation of cell growth or as a response to serum stimulation of quiescent fibroblasts. In this capacity PRC is thought to activate genes encoding the respiratory chain and other growth-related functions through interactions with its target transcription factors. Alternatively, PRC protein levels can be induced in response to severe metabolic stress (red pathway) brought about by nutrient starvation or chemical uncoupling. This is associated with the activation of an antioxidant-sensitive inflammatory/stress program possibly leading to abnormal proliferation or cell death. Genes from this program have been associated with the tumor microenvironment and with cellular senescence. (Panel B) Evidence for elevated PRC protein levels in some solid tumors. A commercial immunoblot from DNA Technologies containing protein samples from several different tumors and the adjacent normal tissue was probed with affinity-purified rabbit anti-PRC antibody or rabbit anti-Tfam. The results show a robust PRC signal in breast, lung and liver tumors compared to modest expression in the surrounding normal tissue. Surprisingly, the PRC signal from breast tumor migrated at a higher molecular mass. The Tfam control was expressed at much reduced levels in several tumor samples compared to the surrounding tissue possibly reflecting the decreased mitochondrial mass associated with the glycolytic nature of many tumors.
