Abstract
Objective
We examined the joint roles of child negative emotionality and parenting in the visual-spatial development of toddlers born preterm or with low birthweights (PTLBW).
Method
Neonatal risk data were collected at hospital discharge, observer- and parent-rated child negative emotionality was assessed at 9-months postterm, and mother-initiated task changes and flexibility during play were observed during a dyadic play interaction at 16-months postterm. Abbreviated IQ scores, and verbal/nonverbal and visual-spatial processing data were collected at 24-months postterm.
Results
Hierarchical regression analyses did not support our hypothesis that the visual-spatial processing of PTLBW toddlers with higher negative emotionality would be differentially susceptible to parenting behaviors during play. Instead, observer-rated distress and a negativity composite score were associated with less optimal visual-spatial processing when mothers were more flexible during the 16-month play interaction. Mother-initiated task changes did not interact with any of the negative emotionality variables to predict any of the 24-month neurocognitive outcomes, nor did maternal flexibility interact with mother-rated difficult temperament to predict the visual-spatial processing outcomes.
Keywords: differential susceptibility, parenting, prematurity, visual-spatial processing
Preterm or low birthweight (PT LBW) births are associated with several negative outcomes including impaired visual-spatial processing (Anderson, Doyle, Callanan, & The Victorian Infant Collaborative Study Group, 2003; O’Reilly et al., 2010). However, recent research and theory suggest that developmental outcomes of children born PT LBW can be influenced by the quality of the early environment and the temperament characteristics of the child (Belsky & Pluess, 2009; Poehlmann, Schwichtenberg, Shlafer, Hahn, Bianchi, & Warner, 2011). Study of PT LBW infants’ visual-spatial processing in the context of parenting is important given findings that positive parent-infant interactions predict healthier cognitive and social development of children who experienced high neonatal risks (e.g., Cohen & Beckwith, 1979; Poehlmann & Fiese, 2001). Moreover, few studies have examined the joint contributions of infant negative emotionality and parenting in predicting visual-spatial processing in young children born PTLBW. In this study, we tested the differential susceptibility hypothesis to determine if infant negative emotionality moderated associations between mothers’ play behaviors at 16-months and children’s 24-month visual spatial outcomes in a sample of children born PTLBW.
1. Maternal Play Behaviors and Cognitive Development in Children Born PTLBW
Maternal flexibility and provision of choices to children during play have been positively associated with term and preterm toddlers’ cognitive development (e.g., Dilworth-Bart, Poehlmann, Miller, & Hilgendorf, 2011; Landry, Smith, Swank, & Miller-Loncar, 2000). Landry and colleagues (2000) found that mothers’ maintaining behaviors (e.g., choice-providing strategies) when their children were two and 3 ½ years old supported general cognitive development both concurrently and when children were 4 ½ years old. Dilworth-Bart et al. (2011) observed similar associations between mothers’ flexibility during play (a variable assessing mothers responsiveness to child initiated task changes) and visual-spatial processing scores in a sample of children born PTLBW. Specifically, higher flexibility at 16-months related to 24-month visual-spatial processing of PTLBW children living in higher, but not lower, SES homes.
Conversely, maternal directiveness during play interactions has been associated with less optimal visual-spatial processing among children born term and preterm. Assel and colleagues observed that maternal directiveness (e.g., providing information but offering little choice) when term and preterm children were two years old had specific negative effects on visual-spatial processing at three years of age (Assel, Landry, Swank, Smith, & Steelman, 2003). In addition, Dilworth-Bart et al. (2011) observed negative associations between maternal directiveness at 16-months postterm and visual-spatial processing at 24-months postterm after controlling for neonatal risks and estimated IQ.
Together, these findings suggest maternal play behaviors that facilitate child choice and exploration during play may be related to more optimal visual-spatial outcomes while behaviors that are directive and restrict child choice may be related to less optimal outcomes. Mothers’ maintaining behaviors or flexibility during play allow children to participate in learning activities while gradually developing independent problem solving skills whereas directive behaviors like mother-initiated task changes may hinder independent exploration and initiation (Landry et al., 2000). However, as previously observed by Dilworth-Bart et al.(2011), zero-order correlations between maternal flexibility and mother-initiated task changes are small and, therefore, may not represent two ends of the same continuum. Rather, small correlations between these constructs may suggest children respond to parenting behaviors differently based on individual characteristics such as temperament.
1.1 Differential Susceptibility to Parenting
The differential susceptibility hypothesis posits that children with certain temperamental characteristics (especially high negative emotionality) are not only more vulnerable to negative aspects of their care giving environments but are also more subject to effects of enriching environments (Belsky, 1997; Belsky, Bakersman-Kranenburg, & van Ijzendoorn, 2007). Interactions between infant temperament characteristics and parenting practices have been observed to influence children’s emerging developmental competencies and problem behaviors (e.g., Belsky & Pluess, 2009; Calkins, 2002; Poehlmann et al., 2011). For example, Stright, Kelley, and Gallagher (2008) found that temperament moderated associations between parenting styles and children’s academic competence, social skills and relationships with teachers and peers. Children with difficult temperaments had better adjustment to school when parenting quality was high and poorer adjustment when parenting quality was low.
Belsky distinguished differential susceptibility from dual risk and contrastive effects (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007). Dual risk occurs when the most vulnerable individuals are disproportionately affected by adverse conditions but are not more affected by positive conditions. Contrastive effects occur when individuals who are more vulnerable are disproportionately affected by both adverse and positive environmental conditions, and individuals who are less vulnerable are affected in the opposite direction so that they fare better in less optimal environmental conditions and display less positive outcomes in more optimal environmental conditions (Belsky et al., 2007).
Poehlmann et al. (2011) observed both dual risk and contrastive effects using the longitudinal sample from which the current subsample was derived. They observed that toddlers born PT LBW who exhibited greater distress demonstrated less delay of gratification when they experienced low maternal positive affect and communication, but maternal positive affect was not related to delay of gratification for PT LBW toddlers who showed lower distress (dual risk). They also found that infants born PT LBW who were rated as more difficult demonstrated lower effortful control in the presence of intrusive, anxious parenting and higher delay of gratification in the presence of positive parenting (contrastive). These associations were not present for infants with less difficult temperaments.
1.2 Purpose
Such evidence suggests children both influence and are influenced by early developmental context, and that developmental context can provide an impetus for children’s biological predispositions to be expressed (Bronfenbrenner & Ceci, 1994). The differential susceptibility model has not been applied to neurocognitive outcomes in children born PTLBW. This study extends previous research by examining joint roles of child negative emotionality and parenting in the visual-spatial development of children born PTLBW. We examined maternal flexibility during play (similar to “maintaining behaviors” discussed above) and mother-initiated task changes (similar to maternal “directiveness” discussed above) in relation to PTLBW toddlers’ emerging visual-spatial processing. The timeframe for our data collection was based on Kopp’s (1982) model of self-regulation that emphasizes the role of temperament around 9-months and parent-child coregulation in toddlerhood as well as prior observations of associations between 16 month parent-child play and 24-month visual-spatial outcomes (Dilworth-Bart, Poehlmann, Hilgendorf, Miller, & Lambert, 2010; Dilworth-Bart et al., 2011). We predicted child negative emotionality at 9-months would moderate associations between maternal play behaviors at 16-months and 24-month postterm visual-spatial processing. We expected that mother-initiated task changes during play would relate to less optimal visual-spatial processing skills when infants were rated high in negative emotionality compared to infants who were less emotionally negative. We also expected that maternal flexibility during play would relate to more optimal visual-spatial processing skills when infants were rated high in negative emotionality compared to infants who were less emotionally negative.
2. Method
2.1 Sample
Data for this study were drawn from a larger longitudinal investigation of early social and physiological processes involved in self-regulation development and their relations to infant-mother attachment and cognitive development in high-risk infants who vary in their level of neonatal medical risk. This longitudinal study followed infants from hospital discharge until they were 2 years (corrected for gestational age) (N = 181).
Participants were recruited into the larger study using five criteria: 1) infants were born less than 36 weeks gestation or weighing < 2500 grams, 2) infants had no known congenital malformations, prenatal drug exposures, or major neurological complications (i.e., no PVL or Grade IV IVH), 3) mothers were at least 17 years old, 4) mothers could read English, and 5) mothers identified themselves as the infant’s primary caregiver. Families were enrolled in the study through 3 hospitals in southeastern Wisconsin, following Institutional Review Board approval from the University of Wisconsin and each of the hospitals. Consent was obtained from the mothers at hospital discharge. If mothers gave birth to multiples, one child was randomly selected to participate in the study. The 181 (97%) study participants came from a total of 186 who signed consent forms at hospital discharge. Data from four of the original 181 families were screened out because we later discovered from our review of infant medical records that a grade IV intraventricular hemorrhage had occurred prior to the infant’s NICU discharge and/or the child was later diagnosed with cerebral palsy. We were unable to calculate participation rates or provide descriptive information about mothers who chose not participate in the study because hospitals would not allow us to be “first contact” with eligible families nor would they provide demographic information about families who chose not to participate.
Mother, child, and dyadic data were collected at hospital discharge as well as at 4-, 9-, 16-, and 24-months postterm. One hundred fifty-nine of the 181 dyads enrolled at hospital discharge completed the 4-month postterm home visit (89%); 153 (86%) completed the 9-month postterm home visit; 151 (85%) completed the 16-month postterm lab visit, and 153 (86%) completed the 24-month postterm lab visit. Although there was 14% attrition between hospital discharge and 24-month assessment, there were no differences in neonatal risk between infants who remained in the study and those whose families discontinued (F(6, 172) = 1.36, p = .23). However, mothers lost to attrition were younger (F(1, 173) = 5.51, p < .05), had less education (F(1, 173) = 5.88, p < .05), and a marginally higher sociodemographic risk index score (F(1, 173) = 3.23, p < .08) (F(7, 167) = 1.90, p < .08). Single mothers (χ2 (1) = 4.68, p < .05) and mothers who were not White (χ2 (1) = 5.57, p < .05) were also more likely to be lost to attrition.
2.1.1 Subsample selection
The final subsample of 63 dyads includes participants who completed the 24-month visual-spatial processing tasks, 16-month play task, 9-month LABTAB and RITQ assessments, and hospital discharge assessment. Visual-spatial processing tasks were added to the larger research battery midway through the 24-month assessment wave as part of a supplemental research project. These subtests were added to the end of the research protocol so the other portions remained unchanged. Children in the subsample did not differ from the full sample in terms of gestational age (t(179) = −0.63, ns), birthweight (t(179) = 0.36, ns), 24-month postterm Abbreviated IQ score (t(151) = −0.36, ns), observer-rated distress (t(146) = 0.44, ns), mother-rated difficult temperament (t(148) = 0.47, ns), or the negativity composite (t(142) = .49, ns). The demographic characteristics of the subsample are provided in Table 1.
Table 1.
Sample description (n = 63)
| Variable | Frequency | Percentage | Mean(SD) | Range | |
|---|---|---|---|---|---|
| Boys | 31 | 49 | |||
| Child Race/Ethnicity | |||||
| Black/African-American | 6 | 9 | |||
| Hispanic/Latino | 1 | 2 | |||
| Mulit-Racial/Multi-Ethnic | 5 | 8 | |||
| White | 51 | 81 | |||
| Gestational Age (weeks) | 31.72 (3.21) | 25 – 36.43 | |||
| <36 weeks | 60 | 95 | |||
| >36 weeks | 3 | 5 | |||
| Birthweight (grams) | 1,757.08 (601.29) | 680 – 3,328.00 | |||
| <750g | 3 | 5 | |||
| 750 – 1499g | 18 | 29 | |||
| 1500 – 2499g | 41 | 65 | |||
| >2500g | 1 | 2 | |||
| Apneaa | 44 | 70 | |||
| Respiratory Distress | 36 | 57 | |||
| Ventilation During NICU Stay | 33 | 52 | |||
| Apnea Monitor at NICU Discharge | 28 | 44 | |||
| NICU Stay > 30 days | 24 | 38 | |||
| Multiple Birth | 12 | 19 | |||
| Chronic Lung Disease | 10 | 16 | |||
| Supplementary Oxygen at NICU Discharge | 9 | 14 | |||
| Gastroesophageal Reflux | 6 | 9 | |||
| Grade I – III IVH | 4 | 6 | |||
| 5 Minute Apgar Score < 6 | 1 | 2 | |||
Complete neonatal risk data were unavailable for one girl participant.
2.2 Measures
2.2.1 General Cognitive Abilities
Children’s general cognitive abilities were estimated with the Abbreviated Battery IQ scale (ABIQ) from the Stanford-Binet Intelligence Scales, 5th edition (SB5; Roid, 2003) and used as a control variable in regression analyses. ABIQ scores are derived from the Object Series/Matrices and Verbal Knowledge routing subtests of the SB5. The Object Series/Matrices routine items provide an estimate of respondents’ cognitive flexibility, fluid reasoning, and abilities to sequence and concentrate (α = .81). The Verbal Knowledge routing subtest items assess word knowledge, verbal fluency, and conceptual thinking (α = .93). The total ABIQ score has a coefficient α of.90, and the correlation with the full scale IQ for 2 to 5 year olds was .81 (Roid, 2003). The ABIQ scale also correlates with the WISC-III (r = .69). Use of the ABIQ along with the visual-spatial processing subscales was deemed appropriate because they rely on different items (Roid, 2003). The SB5 subtests were administered by trained graduate student research assistants who were supervised by a licensed clinical psychologist.
2.2.2 Neonatal Risk
Infant medical records from the child’s NICU stay provided the data for our neonatal health risk variable. We standardized and combined them to create a prematurity composite because infant birthweight and gestational age were highly correlated (r = .87, p < .001). The composite score was reversed so higher scored reflected more prematurity. We then created a neonatal health risk index combining the reversed prematurity composite with 11 other risk variables (scored as 1 if the risk was present; 0 if it was not): grade I – III intraventricular hemorrhage, diagnosis of apnea, respiratory distress syndrome, chronic lung disease, gastroesophageal reflux, multiple birth, whether infants had 5 minute Apgar scores of less than 6, spent more than 30 days in the NICU, were discharged with an apnea monitor, and whether they were still receiving oxygen at hospital discharge (Cronbach’s α = .72). Higher composite scores were indicative of greater neonatal risk.
2.2.3 Child Gender
Gender was coded as a binary variable (0 = girls; 1 = boys).
2.2.4 Infant Temperament
To account for the strengths and weaknesses of parent-reported and observer-rated temperament measures (Rothbart & Bates, 1998), we utilized observer assessment and parental report of infant temperament at 9-months (Time 3).
2.2.4.1 Observer Assessment of Infant Temperament
Tasks from Goldsmith and Rothbart’s (1996) Laboratory Assessment of Temperament (LAB-TAB) were used to assess infant temperament in the home (Kochanska, Coy, Tjbkes, & Husarek, 1998). In this report, we focus on three tasks administered in the home: Unpredictable Mechanical Dog, Plastic Barrier Task, and Colored Block. Tasks were videotaped and coded in accordance with the LAB-TAB manual (Goldsmith & Rothbart, 1996).
For the Unpredictable Mechanical Dog task, we coded intensity of vocal distress and fear response in 10-second intervals. The Plastic Barrier task was coded in 10-second intervals for intensity of vocal distress, facial sadness, bodily sadness, and latency to anger. For the Colored Block task, we coded latency to first look away, duration of orienting to the toy, and duration of manipulating the toy. Tasks were coded by three independent coders, and kappas for each measure ranged from .89 to .92. To reduce these data, we conducted a principal components analysis with varimax rotation on these nine variables and found a two component solution. We labeled the components Distress (4 items, α = .80) and Attention (3 items, α = .62). Items loaded on each component between .66 and .90, with no overlap of items, although two items (latency to anger and intensity of fear response) did not load on either component. The Distress component showed a positive skew because of one extreme outlier, which was dropped. The Attention component was normally distributed. The Attention and Distress components were negatively correlated, r(147) =−.17, p < .05. Only the Distress component was used in the present study.
2.2.4.2 Parent Report of Infant Temperament
The Revised Infant Temperament Questionnaire (RITQ; Carey & McDevitt, 1978) was used to assess maternal perceptions of infant temperament. The RITQ has 95 items that assess the nine dimensions of temperament outlined by Thomas and Chess (1977): activity level, rhythmicity, approach/withdrawal, adaptiveness, intensity, mood, persistence, distractibility, and threshold for stimuli. The RITQ has been used with full-term and preterm infants (e.g. Langkamp, Kim, & Pascoe, 1998) and shows adequate reliability and validity, although the nine dimensions are correlated with each other (e.g., Bohlin, Lindhagen, & Hagekull, 1981). We combined the items from the approach, activity, intensity, negative mood, and adaptability dimensions (after reverse coding where appropriate) into an index of difficult temperament, following previous studies that used the original version of the RITQ (e.g., Bradley & Corwyn, 2008; Stright, Kelley, & Gallagher, 2008). Higher RITQ Difficult scores indicated temperament profiles that reflected less approachability and adaptiveness, and more activity, intensity and negative mood. Scores were normally distributed and Cronbach’s alpha was .80.
2.2.4.3 Negative Emotionality Composite Score
We created a composite score comprised of the standardized and summed LABTAB Distress and RITQ Difficult Temperament scores. Higher scores were indicative of greater negativity.
2.2.5 Maternal Behavior During Play
Mother-child interactions during an approximately 15 minute free play interaction at 16-months postterm were coded for the number of mother-initiated task changes and mothers’ flexibility. Three 2-member coding teams coded the video-taped interaction. Four 2-minute interactions were coded for each tape for a total of 8 coded minutes of play. Segments corresponding to minutes 0 to 2, 3 to 5, 6 to 8, and 9 to 11 were coded for each tape. Scores were summed across the 4 segments. Coding in 2-minute intervals allowed us to gather data about interaction behaviors over an extended time period without fatiguing raters by having them code the full play interaction. We were also unable to code the full 15 minute interaction for several dyads because the interactions concluded early. We stopped coding at the end of the 11th minute of taping because that was the earliest end time for an interaction in our subsample. Inter-rater reliability was calculated for 9 of the 75 dyads (12%) for whom mother-child interaction data were coded. The conservative Fleiss’ κ coefficient was used to establish reliability among the three coding groups for each of the 4 segments (Fleiss, 1971).
The mother-initiated task changes code is conceptually similar to directiveness as defined by Landry and colleagues (2000), in that mothers’ tasks changes reduce the child’s ability to choose activities during play. The mother-initiated task changes code was used to record the total number of times mothers chose a new activity or toy with which to play. Possible scores for this item ranged from 1 task change to 5-or-more changes. Inter-rater reliability was good across the 9 reliability tapes (segment 1 κ = .53, segment 2 κ = .64, segment 3 κ = .70, segment 4 κ = .64).
The mother flexibility code is conceptually similar to the Landry et al. (2000) maintaining code, in that mothers high in flexibility followed the child’s interests during play. This item was used to record mother’s responsiveness to child-initiated task changes (e.g., choosing new toys, adding additional toys to play, or transitioning to a constructive non-play activity like exploring the room). Scores ranged from 1 (does not follow child’s lead during play) to 5 (follows child’s lead and transitions to new activity with ease). Child task changes included. Mothers obtained higher flexibility Inter-rater reliability was moderate to good across the 9 reliability tapes (segment 1 κ = .59, segment 2 κ = .35, segment 3 κ = .48, segment 4 κ = .73).
2.2.6 Visual-Spatial Processing
Visual-spatial processing skills were assessed using the verbal and nonverbal visual-spatial processing subtests of the SB5 (Roid, 2003). The nonverbal subtests include increasingly difficult form-board and pattern activities. The verbal subtests assess knowledge of spatial concepts using position and direction activities. Reliability coefficients for the nonverbal and verbal subtests are .87 and .82, respectively. The correlation between SB5 visual-spatial processing and the abstract reasoning subscale of the SB4 is .69 (Roid, 2003).
2.3 Procedure
Following a family’s enrollment into the study, mothers completed a demographic questionnaire and nurses completed a history of hospitalization form by reviewing the infant’s medical records at the time of infants’ NICU discharge. Child temperament was assessed during a 9-month postterm home visit. Mother-child dyads were invited to the Infant-Parent Interaction Lab at 16-months postterm, and mothers were instructed to play with their toddlers as they did at home prior to engaging in other assessments. Dyads were provided an assortment of age-appropriate toys with which to play, and they were allowed free use of the playroom. Interactions were video-recorded while the experimenter observed through a one-way mirror for the duration of the free-play. Dyads returned to the lab at 24-months postterm and children were administered the SB5 Abbreviated IQ, and the visual-spatial processing subtests as well as other assessments described elsewhere (Poehlmann, Schwichtenberg, Bolt, & Dilworth-Bart, 2009; Poehlmann et al., 2011). Assessments lasted up to 2 hours including rest breaks for the children. Families were compensated with $25 at the 4-month postterm visit, $40 at the 9-month postterm visit, $60 at the 16-month postterm visit, and $80 at the 24-month postterm visit.
2.4 Analysis Plan
Our analyses followed the steps for establishing differential susceptibility (Belsky et al., 2007; Box 1). We calculated means, standard deviations, and scale intercorrelations to test the independence of the susceptibility factor (i.e., temperament) and predictor (i.e., maternal scaffolding) and the association between the susceptibility factor and outcomes. We then established whether child negativity moderated associations between maternal play behaviors and 24-month postterm visual-spatial processing using hierarchical regression models. Associations between control variables (block 1), main effects (block 2), and interactions between parenting behaviors and child negative emotionality (block 3) were examined using separate hierarchical regression models for each maternal play interaction code. All continuous variables were converted to z-scores for the moderation analyses. Twenty-four month Abbreviated IQ and neonatal risk were included as control variables in block 1 because of their potential associations with visual-spatial processing (Dilworth-Bart et al., 2010)1. Interaction terms were entered in block 3 of each model.
Significant interactions were plotted at ± 1 SD of the mean of the moderator variable and compared with prototypical graphs to determine whether the interaction reflected differential susceptibility, dual risk effects, and absence of susceptibility (parallel lines), or contrastive effects (slopes for both groups differed but in opposite directions). We also conducted simple slopes analyses for all regression models that had significant negative emotionality × parenting interactions to determine whether the slopes for children high in negative emotionality were significantly different from zero and steeper than those of children low in negative emotionality (Aiken & West, 1991).
3. Results
3.1 Independence of Susceptibility Factors from Predictor and Outcome Variables
The temperament measures were not associated with maternal flexibility or mother-initiated task changes, nor were the temperament measures associated with the visual-spatial processing variables (r’s ranging from −.15 to .21, all p’s > .05) (Table 2). The lack of significant zero-order correlations between the susceptibility factor (temperament) and both the predictor (maternal play behavior) and the outcomes thus supported the condition that the susceptibility factor be independent from both the predictor and outcome.
Table 2.
Scale intercorrelations, descriptive means, and standard deviations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Mean(SD) | Range | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Abbreviated IQa | 80.52(17.09) | 52 – 121 | |||||||||
| 2 Neonatal Risk | −.04 | 3.10(3.29) | −2.59 – 9.35 | ||||||||
| 3 Distressb | −.07 | .00 | −.03(1.14) | −.54 – 7.23 | |||||||
| 4 Difficult Temperamentc | −.25* | −.08 | .00 | −.06(1.16) | −2.58 – 2.90 | ||||||
| 5 Negativity Composited | −.23 | −.05 | .70*** | .71*** | −.10(1.62) | −3.08 – 7.06 | |||||
| 6 Mother-Initiated Task Changes | −.18 | .00 | .15 | .06 | .15 | 5.41(2.64) | 0 – 12 | ||||
| 7 Maternal Flexibility | .20 | .14 | .01 | −.15 | −.10 | −.08 | 10.54(3.81) | 3 – 19 | |||
| 8 Nonverbal VSPe | .27* | .16 | .21 | −.19 | .02 | −.11 | .07 | 6.98(1.77) | 3 – 11 | ||
| 9 Verbal VSP | .34** | −.05 | .21 | .01 | .15 | −.12 | .03 | .27* | 5.25(2.04) | 3 – 10 |
p < .05;
p < .01;
p < .001
Stanford-Binet Intelligence Scales 5th Edition Abbreviated IQ;
9-month Observer-Rated LAB-TAB Distress;
9-month Mother-Rated RTIQ Difficult Temperament;
Standardized Distress + Difficulty Temperament;
Visual-spatial processing
3.2 Negative Emotionality × Maternal Play Behaviors Predicting 24-month Visual-Spatial Processing
Consistent with testing the differential susceptibility model, we focus on findings regarding the interaction terms in block 3 of the regression models. We observed five interactions suggestive of contrastive effects (Table 3; Figures 1 – 3). Data are organized in the text and tables by temperament characteristics beginning with the more positive maternal play behavior (maternal flexibility) followed by the more negative maternal play behavior (mother-initiated task changes).
Table 3.
Child Negative Emotionality × Maternal Play Behaviors Predicting 24-month Visual-Spatial Processing.
| a. Child Negative Emotionality × Maternal Flexibility | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LABTAB Distress × Maternal Flexibility | RITQ Difficult Temperament × Maternal Flexibility | Negative Emotionality Composite × Maternal Flexibility | ||||||||||||||||||||||||
| Nonverbal VSPa | Verbal VSP | Nonverbal VSP | Verbal VSP | Nonverbal VSP | Verbal VSP | |||||||||||||||||||||
| B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | |||
| 1 ABIQb | .61 | .21 | .34** | .14** | .75 | .24 | .37** | .14* | 1 ABIQ | .61 | .22 | .34** | .13* | .84 | .26 | .38** | .15** | 1 ABIQ | .53 | .22 | .29* | .11 | .77 | .28 | .34** | .11* |
| NN Riskc | .28 | .20 | .16 | −.11 | .23 | −.05 | NN Risk | .25 | .21 | .14 | −.18 | .25 | −.08 | NN Risk | .29 | .21 | .17 | .03 | .26 | .01 | ||||||
| 2 Distd | .12 | .30 | .05 | .00 | .29 | .35 | .11 | .01 | 2 Difff | −.13 | .20 | −.08 | .01 | .18 | .23 | .09 | .01 | 2 Negg | .11 | .14 | .10 | .01 | .30 | .17 | .22 | .05 |
| MFe | −.02 | .24 | −.01 | −.04 | .27 | −.02 | MF | −.09 | .23 | −.05 | .11 | .28 | .05 | MF | −.08 | .24 | −.04 | .15 | .29 | .07 | ||||||
| 3 Dist × MF | .78 | .27 | .38** | .11** | .73 | .32 | .32* | .07* | 3 Diff × MF | .00 | .22 | .00 | .00 | .57 | .25 | .27* | .07* | 3 N × MF | .37 | .17 | .27* | .07* | .59 | .20 | .34** | .11** |
| b. Child Negative Emotionality Maternal Task Changes | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LABTAB Distress × Mother-Initiated Task Changes | RITQ Difficult Temperament × Mother-Initiated Task Changes |
Negative Emotionality Composite × Mother-Initiated Task Changes |
||||||||||||||||||||||||
| Nonverbal VSP | Verbal VSP | Nonverbal VSP | Verbal VSP | Nonverbal VSP | Verbal VSP | |||||||||||||||||||||
| B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | B | SE | B | Δ R2 | |||
| 1 ABIQ | .61 | .21 | .34* | .14** | .75 | .24 | .37** | .14* | 1 ABIQ | .64 | .22 | .35** | .13 | .86 | .26 | .39** | .16 | 1 ABIQ | .53 | 22 | .29* | .11 | .77 | .28 | .34** | .06 |
| NN Risk | .28 | .20 | .16 | −.11 | .23 | −.05 | NN Risk | .22 | .21 | .12 | −.20 | .25 | −.09 | NN Risk | .29 | .21 | .17 | −.03 | .26 | .01 | ||||||
| 2 Dist | .14 | .30 | .06 | .01 | .32 | .34 | .12 | .02 | 2 Diff | −.13 | .20 | −.08 | .03 | .16 | .23 | .08 | .02 | 2 Neg | .12 | .14 | .11 | .01 | .31 | .17 | .23 | .00 |
| MITh | −.11 | .21 | −.06 | −.17 | .24 | −.09 | MIT | −.25 | .21 | −.14 | −.27 | .25 | −.13 | MIT | −.10 | .21 | −.06 | −.27 | .26 | −.13 | ||||||
| 3 Dist × MIT | −.02 | .31 | −.01 | .00 | −.29 | .35 | −.10 | .01 | 3 Dif × MIT | −.22 | .24 | −.12 | .01 | −.13 | .28 | −.06 | .00 | 3 Neg × MIT | −.02 | .19 | −.01 | .00 | −.13 | .24 | −.07 | .01 |
p < .05;
p < .01;
p < .001
Visual-spatial processing;
Abbreviated IQ;
Neonatal Risk;
LABTAB Distress;
Maternal Flexibility;
RITQ Difficult Temperament;
Negativity Composite;
Mother-Initiated Task Changes
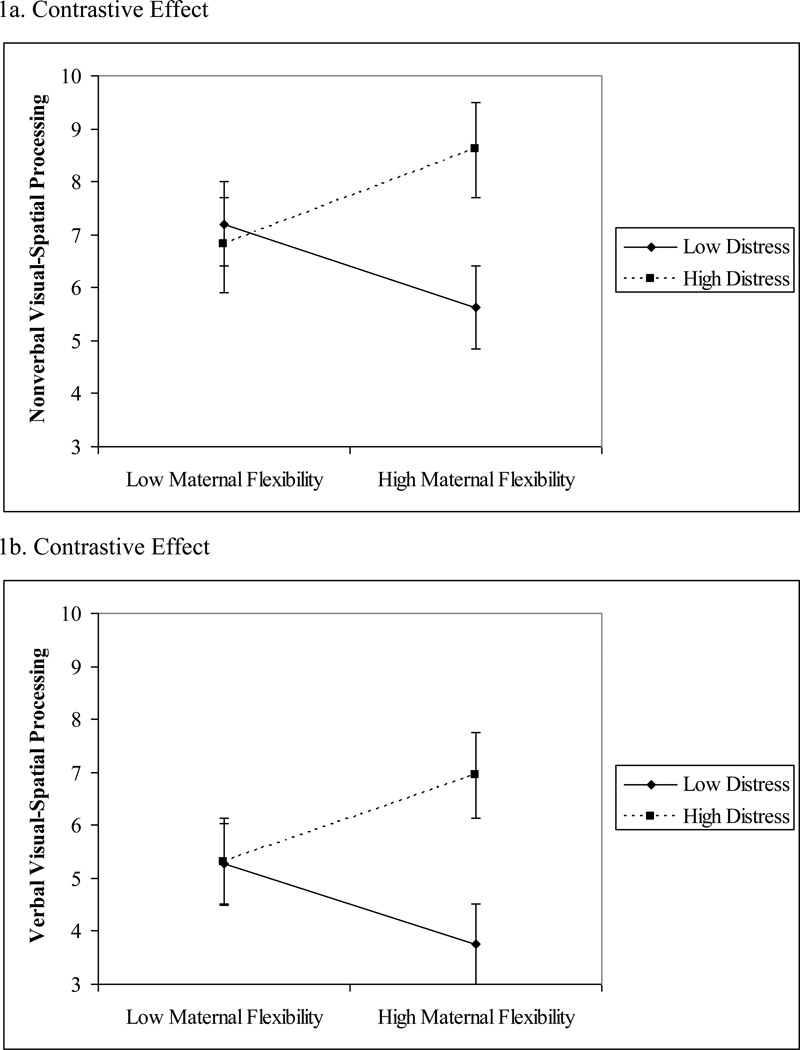
Figure 1.
Observer-Rated Distress (LAB-TAB) × Maternal Flexibility and 24-Month Postterm Nonverbal and Verbal Visual-Spatial Processing
Figure 3.
Negative Emotionality Composite × Maternal Flexibility Interactions and 24-Month Postterm Verbal Visual-Spatial Processing.
3.2.1 Observer-Rated Distress (LABTAB) × Play Behaviors
Maternal flexibility during 16-month play significantly interacted with child distress to predict 24-month nonverbal visual-spatial processing, B = .78, SE = .27, β = .38, p = .006, f2 = .15. The plot of the interaction at ± 1 SD of the moderator mean suggests children with higher observer-rated distress obtained higher 24-month nonverbal visual-spatial processing scores when mothers were more flexible during 16-month play (Figure 1a). However, post-hoc simple slopes analysis indicated the association between maternal flexibility and nonverbal visual-spatial processing for children rated high in distress was nonsignificant, B = .98, SE = .95, β = .45, p = .33, although the beta is in the opposite direction compared to the analysis for children rated low in distress. Instead, more maternal flexibility was related to lower nonverbal visual-spatial processing at 24-months postterm for children rated low in distress, B = −.55, SE = .25, β = −.35, p = .03, f2 = .22. child distress × maternal flexibility interaction term was significant for verbal visual-spatial processing, B = .73, SE = .32, β = .32, p = .02, f2 = .09. Similar to nonverbal visual-spatial processing, children with higher observer-rated distress obtained higher 24-month verbal visual-spatial processing scores when mothers were more flexible during 16-month play (Figure 1b). However, post-hoc testing indicated the association between maternal flexibility and verbal visual-spatial processing was not statistically significant at high, B = .69, SE = 1.09, β = .28, p = .54, or low, B = −.30, SE = .22, β = −.22, p = .19, levels of distress.
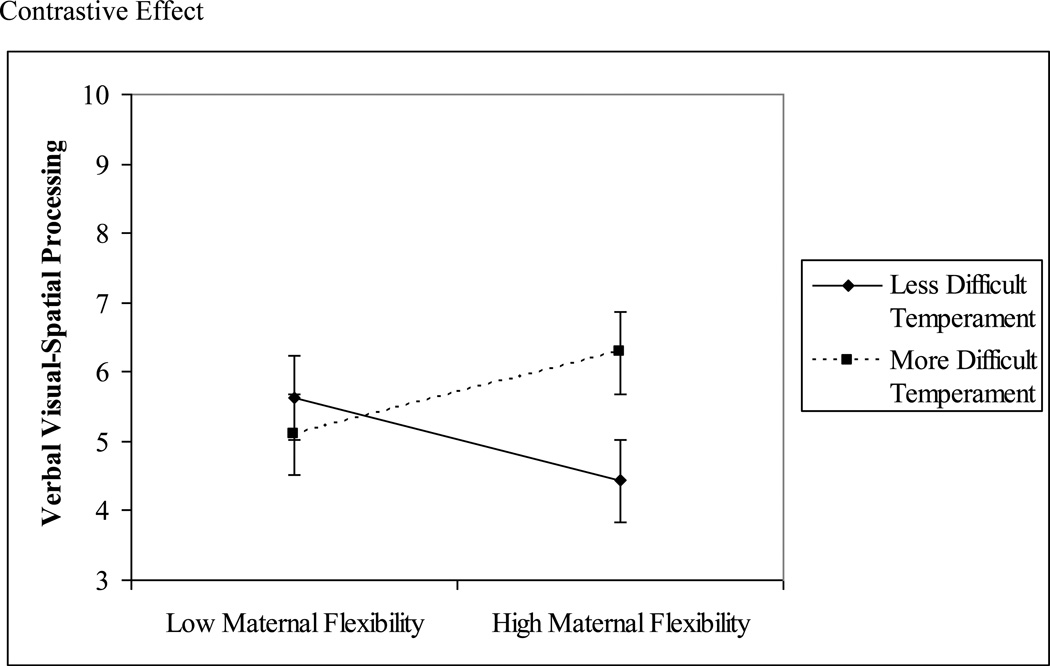
3.2.2. Mother-Rated Difficult Temperament (RITQ) × Play Behaviors
Maternal flexibility during 16-month play significantly interacted with RITQ Difficult Temperament scores to predict 24-month verbal visual-spatial processing in block 3, B = .56, SE = .25, β = .27, p = .03, f2 = .10 (Table 3). Children with higher mother-rated difficult temperament obtained higher verbal visual-spatial processing scores when mothers were more flexible (Figure 2). However, post-hoc analyses revealed no significant associations between maternal flexibility and verbal visual-spatial processing at either high B = .57, SE = .37, β = .30, p = .15, or low, B = −.32, SE = .40, β = −.14, p = .43, levels of mother-rated difficult temperament.
Figure 2.
Mother-Rated Difficult Temperament × Maternal Flexibility Interactions and 24-Month Postterm Verbal Visual-Spatial Processing.
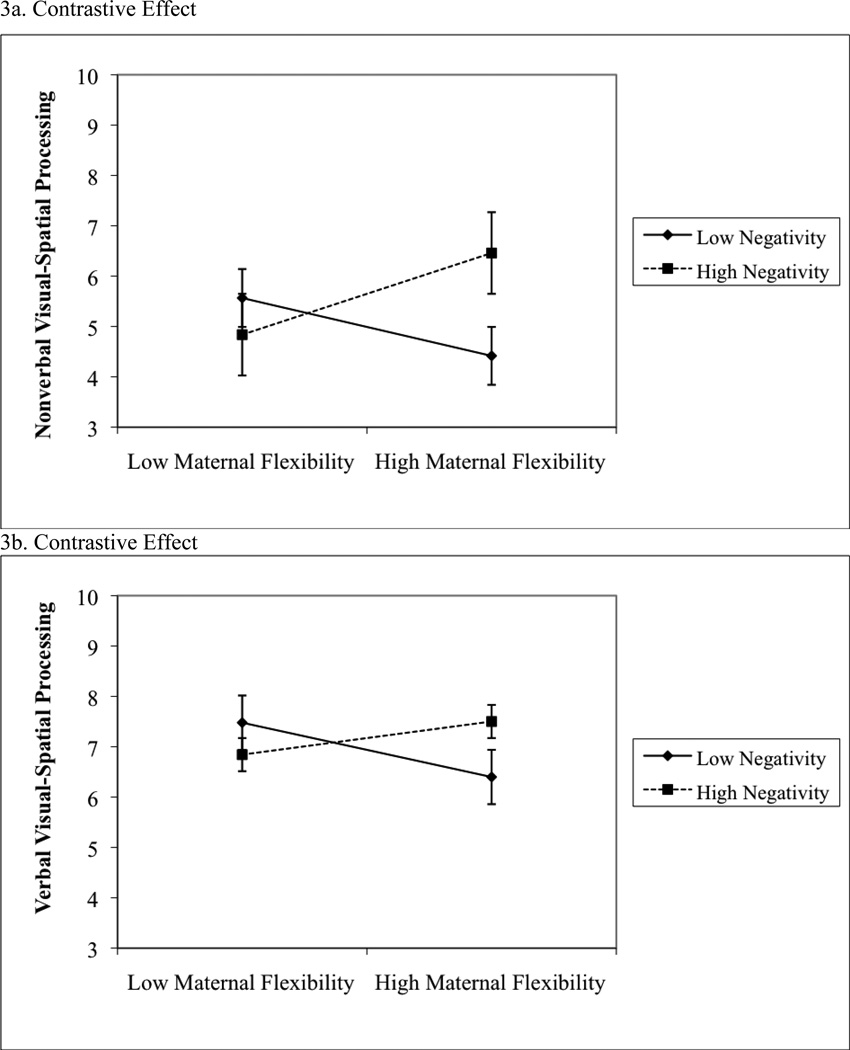
3.3.3 Negativity Composite × Play Behaviors
Maternal flexibility during 16-month play interacted with the negativity composite to predict 24-month nonverbal visual-spatial processing, B = .37, SE = .17, β = .27, p = .03, f2 = .09. Children with higher negativity composite scores obtained higher nonverbal visual-spatial processing scores when mothers were more flexible (Figure 3a). However, post-hoc analyses revealed no statistically significant associations between maternal flexibility and nonverbal visual-spatial processing at high, B = .71, SE = .44, β = .41, p = .15, or low, B = .75, SE = .74, β = .30, p = .34, levels of the negativity composite.
Second, maternal flexibility significantly interacted with the negativity composite score to predict verbal visual-spatial processing, B = .78, SE = .20, p < .001, f2 = .27. The plot of the interaction suggests a contrastive effect in which children with higher negativity composite scores obtained higher verbal visual-spatial processing scores when mothers were more flexible (Figure 3b). Post hoc analyses revealed a significant association between maternal flexibility and verbal visual-spatial processing at high levels of the negativity composite, B = 1.97, SE = .51, β = .77, p = .006, f2 = 2.70. Although the sign was in the opposite direct, the association was nonsignificant at low levels of the negativity composite (B = −1.40, SE = 1.01, β = −.41, p = .20).
None of the negative emotionality indicators significantly interacted with mother-initiated task changes to predict either nonverbal or verbal visual-spatial processing (all p’s > .05). Similarly, RITQ scores did not interact with maternal flexibility to predict nonverbal visual-spatial processing (p > .10) (Table 3).
4. Discussion
This study examined the associations between child negative emotionality, mothers’ play behaviors, and the visual-spatial processing of children born preterm or with low birthweights (PTLBW). We extended previous research about the associations between parenting behaviors and child neurocognitive outcomes (e.g., Landry et al., 2000) by examining the potential contributions of child temperament to parent-child play interactions. However, our analyses did not support the hypothesis that the visual-spatial processing of children born preterm or with low birth weights with higher negative emotionality would be differentially susceptible to parenting behaviors during play. Analyses revealed associations between maternal flexibility during 16-month play (index of more positive play behaviors) and 24-month visual-spatial processing, but we did not observe any associations between visual-spatial processing and mother-initiated task changes (index of more negative play behaviors).
The plots of the significant interactions suggested contrastive effects in which children with higher negative emotionality displayed higher visual-spatial processing in the presence of greater maternal flexibility. Two significant interactions were supported by statistically significant post hoc analyses. First, simple slopes analyses indicated a statistically significant effect of higher scores on the negative emotionality composite on verbal visual-spatial processing. This finding is consistent with the premise of the differential susceptibility hypothesis that children with greater negative emotionality could be more susceptible to the positive aspects of the developmental context (Belsky et al., 2007). However, this finding does not provide conclusive evidence of differential susceptibility given the absence of a significant negative emotionality × negative play behavior (i.e., mother-initiated task changes) interaction.
Second, simple slopes analysis indicated that children rated low in distress at 9-months had less optimal 24-month nonverbal visual-spatial processing when mothers were more flexible compared to children whose mothers were less flexible. Although the betas were in the opposite direction for children rated high in distress at 9-months, post-hoc analyses revealed no statistically significant association between maternal flexibility and visual-spatial processing for easily distressed children.
These findings suggest that differential susceptibility in PTLBW toddlers may be limited to the development of behavior problems rather than extending to their visual-spatial skills. In a previous analysis with the larger sample from which the current sample was derived, Poehlmann et al., 2011 found that infants rated as high in distress were more likely to develop behavior problems at 24 months in the context of less optimal early parenting but less likely to develop such problems in the context of optimal early parenting. However, differential susceptibility was not found in relation to PTLBW children’s emerging effortful control skills (Poehlmann et al., 2011). Future studies should continue to examine the differential susceptibility model with other high-risk populations to determine the extent and limits of this model in relation to child and family risks.
High risk infants may have different interactional needs than lower risk infants, challenging what we typically conceptualize as more and less optimal parental scaffolding behaviors. For example, in the present study, PTLBW infants with lower observer ratings of distress at 9-months may have needed more maternal structure and consistency rather than flexibility and choice during play at 16 months. This interpretation is consistent with Landry et al.’s (2000) findings that maternal directiveness facilitated term and preterm children’s general cognitive development as long as the directive behaviors were appropriately matched to the child’s need for support.
In contrast, it is also possible that mothers of children with low distress ratings may have been less adept at reading their children’s subtle affective cues and structuring the play interaction in a way that would have been most beneficial for the children, as has been observed in other studies of PTLBW children (Macey, Harmon, & Easterbrooks, 1987; Poehlmann & Fiese, 2001). However, data focusing directly on maternal sensitivity to child affective cues would be needed, especially for those children rated low in distress to novelty at 9-months. This interpretation is also supported by transactional developmental theory, in that developmental outcomes appear to result from dynamic interactions between children and their families over time and that both child characteristics and parental behaviors are important contributors (Seifer & Sameroff, 1987).
We did not observe significant main effects for either the parenting or temperament variables. This finding suggests that the previously observed direct associations between parenting and temperament and child outcomes (e.g., Niccols & Feldman, 2006; Tamis-LeMonda et al., 2009) may be specific to toddlers’ problem behaviors and not extend to the early visual-spatial processing of children born PTLBW. However, our finding of significant distress × flexibility interaction effects on the visual-spatial processing index supports the notion that behaviors such as maternal flexibility must increase or decrease relative to children’s characteristics in order to benefit child outcomes.
The study subsample was relatively small for identifying moderation and participants all experienced relatively high levels of neonatal risk. The small sample size also precludes us from analyzing differences between children born as singletons versus those born as multiples. We are similarly limited in our ability to discuss the implications of our findings to higher SES-risk and/or ethnically diverse children because families experiencing greater sociodemographic risks and mothers who were not white were differentially lost to attrition. Nevertheless, these findings highlight ways family processes interact with child characteristics to promote or inhibit visual-spatial processing and suggest the need for continued research with a larger, more sociodemographically diverse sample.
Research Highlights.
We examined child negativity, maternal play and PTLBW toddlers’ VSP.
Flexible play positively related to verbal VSP when child negativity was high.
Flexible play negatively related to nonverbal VSP when child negativity was low.
Differential susceptibility in PTLBW toddlers may be limited to development of behavior problems.
Acknowledgments
This research was funded by the University of Wisconsin and the National Institutes of Health (MD000506 andHD044163).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We ran these analyses with and without the neonatal risk control variable at the suggestion of an anonymous reviewer. Removal of neonatal risk did not substantively alter our findings.
References
- Aiken L, West S. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Anderson P, Doyle LW Victorian Infant Collaborative, S. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. Jama-Journal of the American Medical Association. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. PMid:12824207. [DOI] [PubMed] [Google Scholar]
- Assel M, Landry S, Swank P, Smith K, Steelman L. Precursors to mathematical skills: Examining the roles of visual-spatial skills, executive processes, and parenting factors. Applied Developmental Science. 2003;7(1):27–38. [Google Scholar]
- Belsky J. Theory testing, effect-size evaluation, and differential susceptibility to rearing influence: The case of mothering and attachment. Child Development. 1997;68(4):598–600. [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16(6):300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bohlin G, Lindhagen K, Hagekull B. Validity of parental reports. Infant Behavior & Development. 1981;7(1):77–92. http://www.sciencedirect.com/science/journal/01636383. [Google Scholar]
- Bradley RH, Corwyn RF. Infant temperament, parenting, and externalizing behavior in first grade: a test of the differential susceptibility hypothesis. Journal of Child Psychology and Psychiatry. 2008;49(2):124–131. doi: 10.1111/j.1469-7610.2007.01829.x. http://www3.interscience.wiley.com/journal/117960395/home. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci S. Nature-Nurture reconceptualized in developmental perspective: A bioecological model. Psychological Review. 1994;101(4):568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Does aversive behavior during toddlerhood matter? The effects of difficult temperament on maternal perceptions and behavior. Infant Mental Health Journal. 2002;23(4):381–402. [Google Scholar]
- Carey WB, McDevitt SC. Revision of the infant temperament questionnaire. Pediatrics. 1978;61:735–739. http://pediatrics.aappublications.org/ [PubMed] [Google Scholar]
- Chess S, Thomas A. Temperamental individuality from childhood to adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 1977;16(2):218–226. doi: 10.1016/s0002-7138(09)60038-8. [DOI] [PubMed] [Google Scholar]
- Cohen S, Beckwith L. Preterm infant interactions with the caregiver in the first year of life and competence at age two. Child Development. 1979;50(3):767–776. [PubMed] [Google Scholar]
- Dilworth-Bart J, Poehlmann J, Hilgendorf A, Miller K, Lambert H. Maternal scaffolding and preterm toddlers’ visual-spatial processing and emerging working-memory. Journal of Pediatric Psychology. 2010;35(2):209–220. doi: 10.1093/jpepsy/jsp048. PMid:19505998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth-Bart J, Poehlmann J, Miller K, Hilgendorf A. Do mothers’ play behaviors moderate the associations between socioeconomic status and 24-month neurocognitive outcomes? Journal of Pediatric Psychology. 2011;36(3):289–300. doi: 10.1093/jpepsy/jsq064. PMid:20656763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Measuring nominal scale agreement among many raters. Psychological Bulletin. 1971;76(5):378–382. [Google Scholar]
- Goldsmith HH, Rothbart M. In: The laboratory temperament assessment battery (LABTAB): Prelocomotor version 3.0. Goldsmith Hill H., PhD, editor. Madison, WI: Personality Development Laboratory, Department of Psychology, University of Wisconsin; 1996. [Google Scholar]
- Kochanska G, Coy K, Tjebkes T, Husarek SJ. Individual differences in emotionality in infancy. Child Development. 1998;64(2):375–390. [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18(2):199–214. [Google Scholar]
- Landry S, Smith KE, Swank PR, Miller-Loncar CL. Early maternal and child influences on children's later independent cognitive and social functioning. Child Development. 2000;71(2):358–375. doi: 10.1111/1467-8624.00150. [DOI] [PubMed] [Google Scholar]
- Langkamp DL, Kim Y, Pascoe JM. Temperament of preterm infants at 4 months of age: Maternal ratings and perceptions. Journal of Developmental and Behavioral Pediatrics. 1998;19(6):391–396. doi: 10.1097/00004703-199812000-00001. http://journals.lww.com/jrnldbp/pages/default.aspx. [DOI] [PubMed] [Google Scholar]
- Macey TJ, Harmon RJ, Easterbrooks MA. Impact of premature birth on the development of the infant in the family. Journal of Consulting and Clinical Psychology. 1987;55(6):846–852. doi: 10.1037//0022-006x.55.6.846. [DOI] [PubMed] [Google Scholar]
- Niccols A, Feldman M. Maternal sensitivity and behaviour problems in young children with developmental delay. Infant and Child Development. 2006;15(5):543–554. [Google Scholar]
- O'Reilly M, Vollmer B, Vargha-Khadem F, Neville B, Connelly A, Wyatt J, et al. Ophthalmological, cognitive, electrophysiological and MRI assessment of visual processing in preterm children without major neuromotor impairment. Developmental Science. 2010;13(5):692–705. doi: 10.1111/j.1467-7687.2009.00925.x. PMid:20712735. [DOI] [PubMed] [Google Scholar]
- Poehlmann J, Fiese BH. Parent-infant interaction as a mediator of the relation between neonatal risk status and 12-month cognitive development. Infant Behavior & Development. 2001;24(2):171–188. [Google Scholar]
- Poehlmann J, Schwichtenberg AJM, Bolt D, Dilworth-Bart J. Predictors of depressive symptom trajectories in mothers of preterm or low birth weight infants. Journal of Family Psychology. 2009;23(5):690–704. doi: 10.1037/a0016117. PMid:19803605 PMCid:2791691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J, Schwichtenberg AJM, Shlafer RJ, Hahn E, Bianchi J-P, Warner R. Emerging self-regulation in toddlers born preterm or low birth weight: Differential susceptibility to parenting. Development and Psychopathology. 2011;23(1):177–193. doi: 10.1017/S0954579410000726. PMid:21262047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid G. Technical Manual. Fifth Edition. Itasca, IL: Riverside Publishing; 2003. Stanford-Binet Intelligence Scales. [Google Scholar]
- Rothbart M, Bates J. Temperament. In: William D, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional and personality development. 5th ed. Hoboken, NJ: John Wiley & Sons, Inc; 1998. pp. 105–176. [Google Scholar]
- Seifer R, Sameroff AJ. Multiple determinants of risk and invulnerability. In: Anthony EJ, editor. Invulnerable child. New York, NY: Guilford Press; 1987. pp. 51–69. [Google Scholar]
- Stright AD, Kelley K, Gallagher KC. Infant temperament moderates relations between maternal parenting in early childhood and children's adjustment in first grade. Child Development. 2008;79(1):186–200. doi: 10.1111/j.1467-8624.2007.01119.x. PMid:18269517. [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Briggs RD, McClowry SG, Snow DL. Maternal control and sensitivity, child gender, and maternal education in relation to children's behavioral outcomes in African American families. Journal of Applied Developmental Psychology. 2009;30(3):321–331. doi: 10.1016/j.appdev.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]