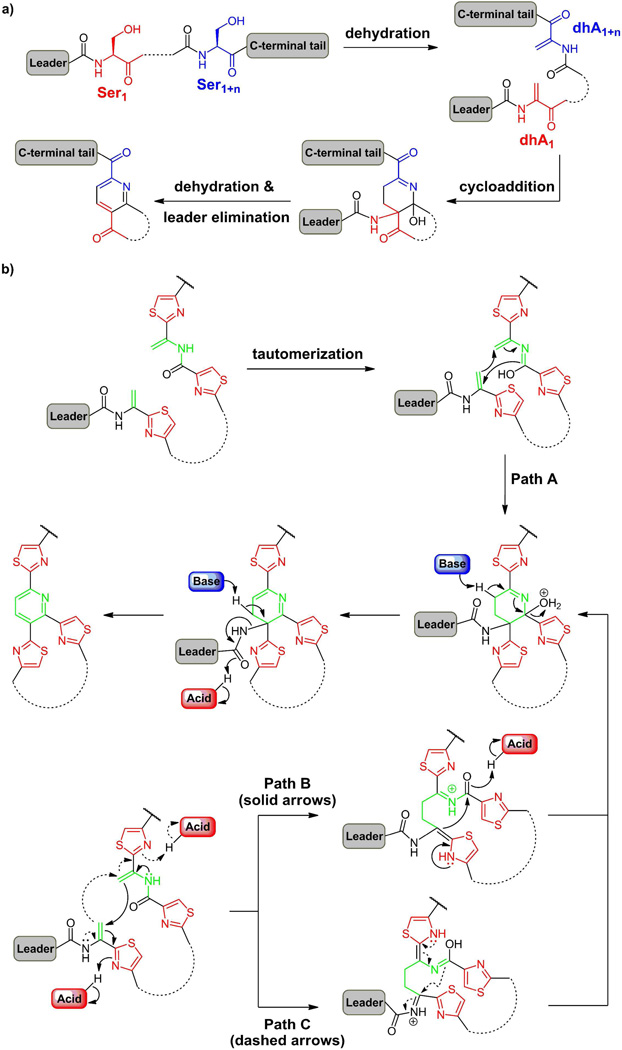
Figure 11.
(a) The Bycroft-Floss hypothesis suggests pyridine ring formation from two dehydroalanine residues; (b) Construction of the central trithiazolyl pyridine core from two dhA residues at a late stage of antibiotic scaffold maturation after twin posttranslational modifications have already occurred; (b) possible concerted vs stepwise mechanisms for an aza-Diels-Alder cyclization, followed by dehydration to yield a dihydropyridine with the upstream leader peptide attached. Aromatizing elimination would yield the pyridine in the majority of cases while a two electron reduction would give the tetrahydropyridine core seen in thiostrepton with the leader peptide still in place.