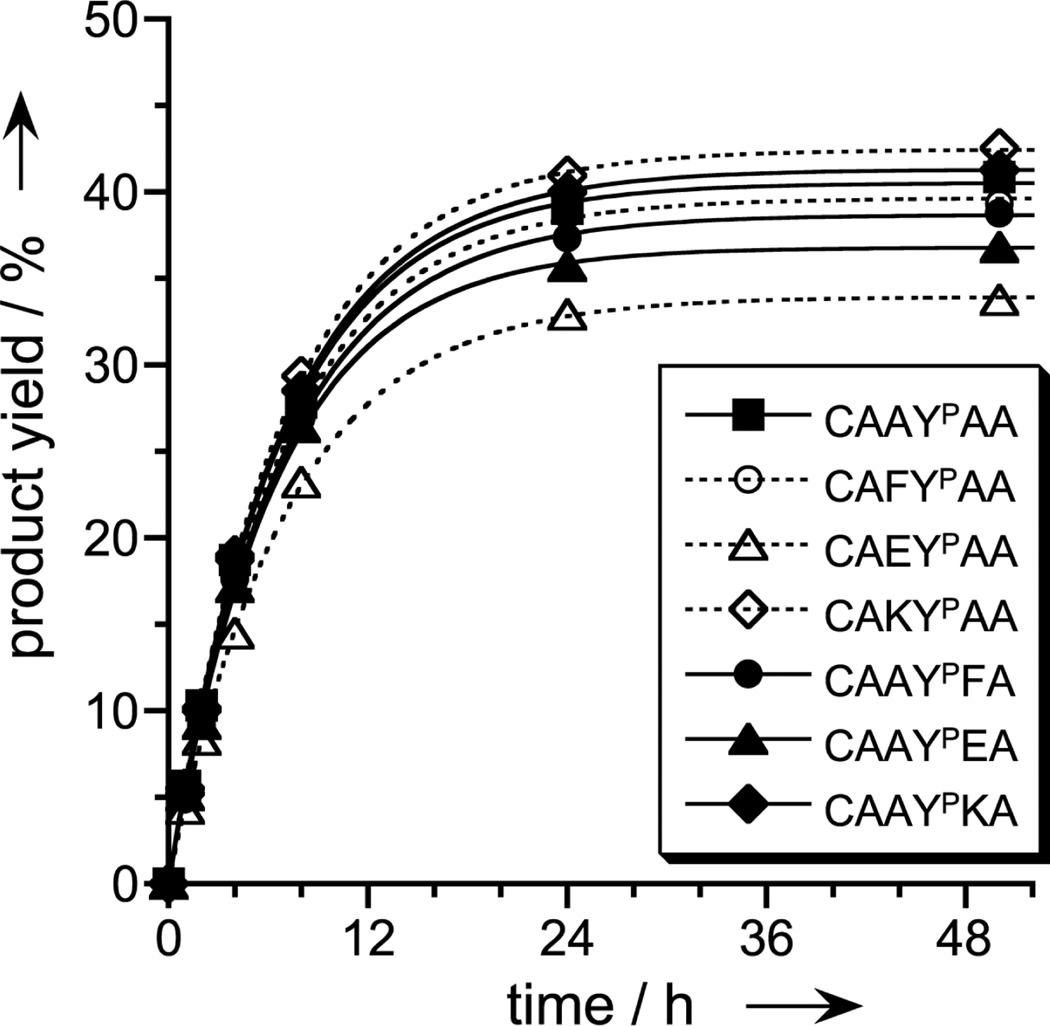
Figure 3.
Sequence generality for the phosphopeptide substrate. Covalent modification by 8VP1 was examined with DNA-anchored hexapeptide substrate CAAYPAA and several illustrated sequence variants, for which one amino acid adjacent to YP (on either side) was changed to one of F (hydrophobic), E (negatively charged), or K (positively charged). Experiments were performed as in Figure 2. Each peptide was attached to the DNA anchor via the N-terminal cysteine side chain, which enables inclusion of K within the sequence and also allows cleavage of the peptide from the anchor by DTT reduction.