Figure 5. Genetic evidence that MN30 inactivates A3G by binding C321.
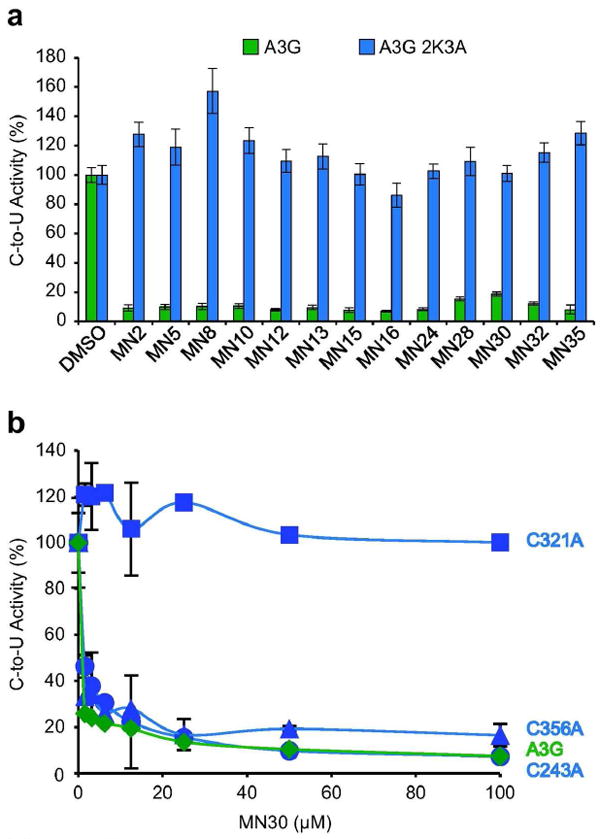
a) Full-length A3G-2K3A completely resists inhibition by all tested catechol series inhibitors. The mean and SD of triplicate deaminase assays with 50 μM compound, 0.0675 μM A3G, 0.33 μM ssDNA, and excess UDG are shown relative to the DMSO only controls.
b) A single amino acid substitution C321A defines the binding site in A3G for catechol series inhibitors. Wildtype A3G and the indicated single alanine derivatives were purified as described in the methods and Supplementary Figure S1 and assayed in parallel with varying concentrations of MN30. Data from triplicate assays were normalized to the DMSO only controls, and the relative mean activities are presented with SD.
