Abstract
Basiliskamide A is an antifungal polyketide natural product isolated by Andersen and co-workers from a Bacillus laterosporus isolate, PNG-276. A nine-step enantioselective synthesis of basiliskamide A is reported, starting from commercially available β-hydroxy ester 7. The synthesis features a highly diastereoselective mismatched double asymmetric δ-stannylallylboration reaction of aldehyde 5 with the bifunctional allylborane reagent 4.
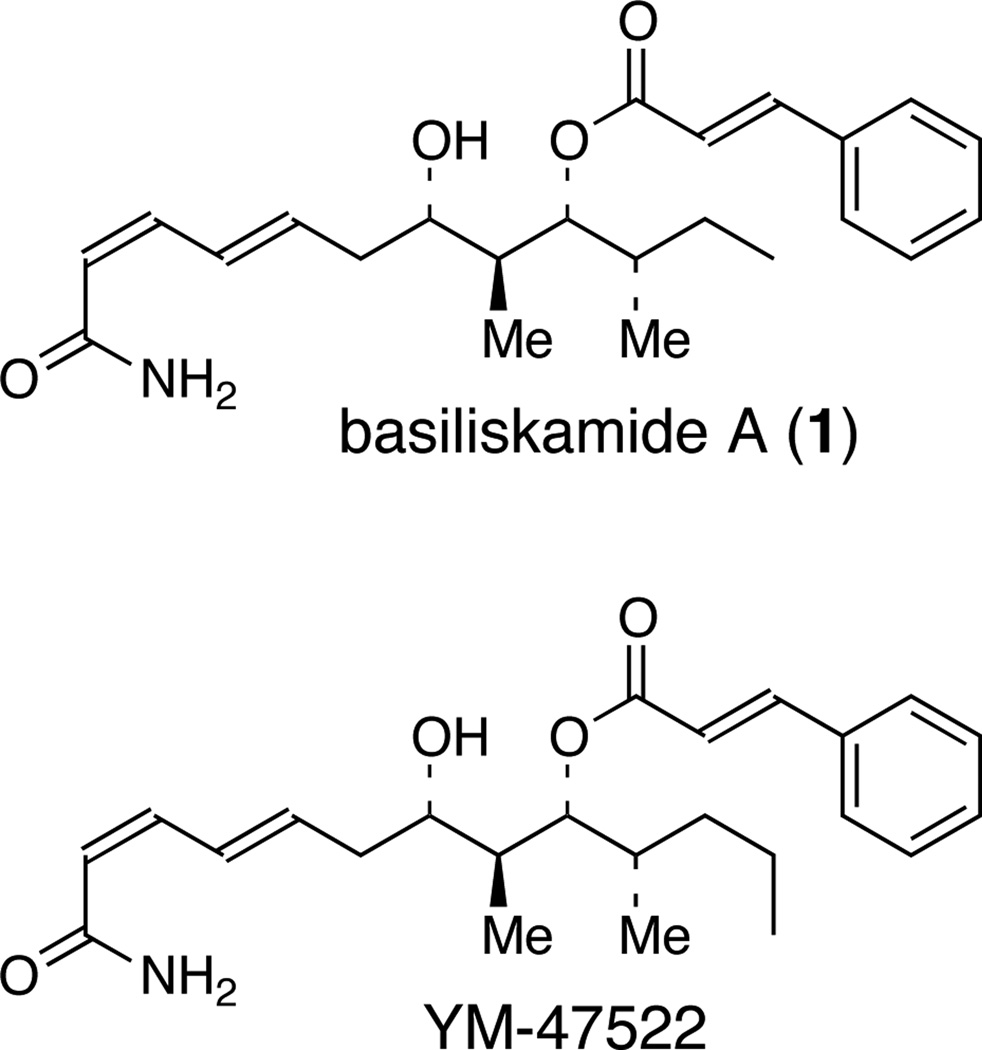
Basiliskamide A (1) is a polyketide natural product isolated by Andersen and co-workers from a Bacillus laterosporus isolate, PNG-276.1 The structure of 1 was established by extensive NMR experiments and by comparison of its spectroscopic properties to that for the known one carbon homologation analog, YM-47522 (Figure 1). The relative and absolute configuration assignments for basiliskamide A were subsequently verified by chemical synthesis.2
Figure 1.
Structures of basiliskamide A and YM-47522.
Basiliskamide A (1) displays biological activity against C. albicans and A. fumigatus with MIC values of 1.0 µg/mL and 2.5 µg/mL, respectively. Intriguingly, in spite of the close structural similarities between basiliskamide A (1) and YM-47522 (Figure 1), the biological activity profile of YM-47522 is quite different, with MIC values against C. albicans and A. fumigatus of 25 µg/mL and >50 µg/mL, respectively. Furthermore, studies with seven fresh clinical isolates of C. albicans indicated that basiliskamide A has activity comparable to the oxo polyene macrolide antibiotic amphotericin B, with both compounds having identical MICs (0.5 µg/mL) against each of the seven clinical isolates. More importantly, basiliskamide A displayed only minimal cytotoxicity against human diploid fibroblast cells at a concentration of 100 µg/mL, while amphotericin B destroyed all the cells at the same concentration.1
This interesting biological profile has inspired the development of several total syntheses of basiliskamide A (1).2 The shortest of the three current syntheses requires 14 linear steps (16 steps total) from commercially available starting materials. Several steps in each synthesis are devoted to protecting group manipulations or to reduction or oxidation reactions needed to adjust the oxidation state of increasingly advanced intermediates. Each of the reported syntheses of 1 also utilized a Stille coupling of vinyl iodide intermediates, which were prepared via Takai olefination of appropriate aldehyde precursors.2, 3a,b One drawback associated with Takai olefination is that a mixture of E/Z olefin isomers is often obtained, with the ratio of the two isomers depending on structural features of the aldehyde substrate.2, 3c–e With the goals to develop an efficient synthesis of 1, to demonstrate the synthetic utility of the highly diastereoselective mismatched double asymmetric δ-stannylallylboration reaction of reagent 4,4, 5, 6 and ultimately to use this synthesis to gain further insight into the structure-activity relationships of these natural products, we have developed and report herein a nine-step, enantio- and highly diastereoselective synthesis of (−)-basiliskamide A (1).
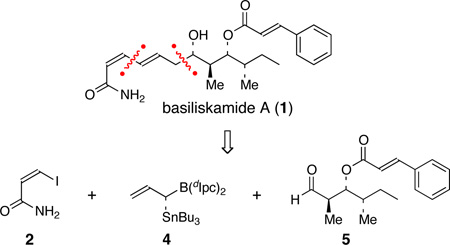
As summarized in Figure 2, we envisioned that basiliskamide A (1) could be assembled via a Stille coupling7 between the known vinyl iodide 28 and vinyl stannane 3. The δ-stannyl-homoallylic alcohol moiety embedded in vinyl stannane 3 provides a perfect platform to explore the mismatched double asymmetric δ-stannylallylboration reaction of aldehyde 5 with our recently disclosed allylborane reagent 4.4 Aldehyde 5 would be obtained from the known homoallylic alcohol 6,9 which in turn would be prepared from the commercially available β-hydroxy ester (S)-7 according to published procedures.
Figure 2.
Retrosynthetic analysis of basiliskamide A (1).
Homoallylic alcohol 6 was synthesized in three steps according to known procedures, starting from β-hydroxy ester (S)-7 (Scheme 2).9 Hydrogenation of the olefin unit in 6 under standard conditions (Pd/C, H2, MeOH-EtOAc) provided, unexpectedly, the deprotected diol 9 in 92% yield (Scheme 1).10 When EtOAc was used as the reaction solvent, alcohol 8 with the primary TBS ether intact was obtained in 63% yield; however, a significant amount of the ketone byproduct 10 (23%) was also obtained. After a brief screening of reaction conditions, the formation of ketone 10 was minimized by adding NaHCO3 to the hydrogenation reaction and by shortening the reaction time. Under optimized conditions, alcohol 8 was obtained in 81% yield from alcohol 6.
Scheme 2.
Total Synthesis of Basiliskamide A (1)
Scheme 1.
Optimization of Hydrogenation of Alcohol 6
Acylation of alcohol 8 with (E)-cinnamoyl chloride (11) provided ester 12 in 63% yield (92% based on recovered starting material, Scheme 2). Deprotection of the primary TBS ether of ester 12 proved to be challenging (see Table 1). When this deprotection was attempted using TsOH in a THF-MeOH solvent mixture, a 1:1 mixture of alcohol 13 and the acyl transfer isomer 14 were obtained (Table 1, entry 1). Treatment of 12 with TBAF in THF again provided a 1:1 mixture of alcohols 13 and 14 (Table 1, entry 2). Similar results were also obtained when TBAF buffered with HOAc was used (Table 1, entry 3). However, when 12 was treated with TASF in a DMF-H2O mixture,11 a 9:1 mixture of 13 and 14 was generated, from which alcohol 13 was obtained in 79% yield after chromatographic purification (Table 1, entry 4).
Table 1.
Optimization of the Deprotection of TBS Ether 12
 | |||
|---|---|---|---|
| entry | conditions | 13:14 a | yield (13) b |
| 1 | TsOH, THF/MeOH, rt, 8 h | 1:1 | 43% |
| 2 | TBAF, THF, rt, 8 h | 1:1 | 47% |
| 3 | TBAF/HOAc, THF, rt, 8 h | 1:1 | 41% |
| 4 | TASF, DMF/H2O, rt, 8 h | 9:1 | 79% |
Based on 1H NMR analysis of the crude reaction mixture.
Yield of isolated desired alcohol 13.
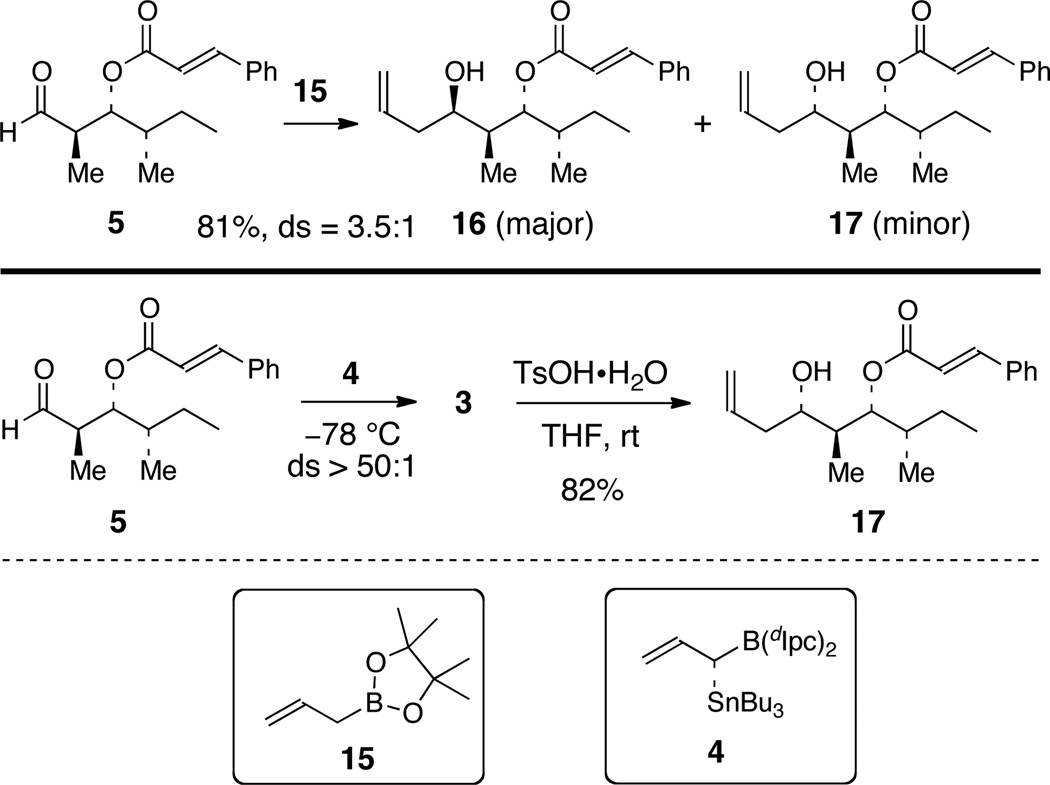
Oxidation of primary alcohol 13 with the Dess-Martin periodinane reagent12 provided aldehyde 5, which was used directly in the subsequent reaction. The intrinsic diasteofacial selectivity of 5 was accessed by subjecting 5 to an allylboration reaction with the achiral pinacol allylboronate 15 (Scheme 3). This reaction provided a 3.5:1 mixture of homoallylic alcohol 16 and 17, favoring the expected Felkin adduct 16. On the other hand, the mismatched double asymmetric δ-stannylallylboration of aldehyde 5 with allylborane 4, prepared as described previously,4a gave homoallylic alcohol 3 with >50:1 diastereoselectivity and in 71% overall yield from 13. Protodestannylation of 3 under acidic conditions (TsOH•H2O) provided alcohol 17 in 82% yield, which matched the minor product obtained from allylboration of aldehyde 5 with allylboronate 15 (Scheme 3).
Scheme 3.
Allylboration Studies of Aldehyde 5
Finally, Pd-catalyzed Stille coupling7 of vinyl stannane 3 with vinyl iodide 28 provided (−)-basiliskamide A (1) in 68% yield. The spectroscopic data (1H NMR, 13C NMR, [α]D) of synthetic (−)-basiliskamide A were in excellent agreement with the data previously reported for the natural product.1, 2
In conclusion, the enantioselective total synthesis of (−)-basiliskamide A was completed in 9 steps (longest linear sequence, 10 steps total) from commercially available starting materials. The brevity of this synthesis was facilitated by the mismatched double asymmetric δ-stannylallylboration reaction of aldehyde 5 with allylborane 44a that provided homoallylic alcohol 3 with outstanding stereochemical control (>50:1). The vinyl stannane unit in 3 was used directly in a Stille coupling reaction to complete the total synthesis of (−)-basiliskamide A. Application of this methodology to the synthesis of other related polyketide natural products will be reported in due course.
Supplementary Material
Acknowledgment
Financial support provided by the National Institutes of Health (GM038436) and Eli Lilly (for a predoctoral fellowship to M.C.) is gratefully acknowledged.
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Barsby T, Kelly MT, Andersen RJ. J. Nat. Prod. 2002;65:1447. doi: 10.1021/np0201321. [DOI] [PubMed] [Google Scholar]
- 2.(a) Lipomi DJ, Langille NF, Panek JS. Org. Lett. 2004;6:3533. doi: 10.1021/ol048574m. [DOI] [PubMed] [Google Scholar]; (b) Dias LC, Gonçalves CCS. Adv. Synth. Catal. 2008;350:1017. [Google Scholar]; (c) Dias LC, Gonçalves CCS. J. Braz. Chem. Soc. 2010;21:2012. [Google Scholar]; (d) Yadav JS, Rao PP, Reddy MS, Prasad AR. Tetrahedron Lett. 2008;49:5427. [Google Scholar]
- 3. Takai K, Nitta K, Utimoto K. J. Am. Chem. Soc. 1986;108:7408. doi: 10.1021/ja00279a068. Evans DA, Black WC. J. Am. Chem. Soc. 1993;115:4497.. For recent examples: Palimkar SS, Uenishi J. Org. Lett. 2010;12:4160. doi: 10.1021/ol101753y. Li P, Li J, Arikan F, Ahlbrecht W, Dieckmann M, Menche D. J. Org. Chem. 2010;75:2429. doi: 10.1021/jo100201f. Crimmins MT, Haley MW, O’Bryan EA. Org. Lett. 2011;13:4712. doi: 10.1021/ol201920j..
- 4.(a) Chen M, Ess DH, Roush WR. J. Am. Chem. Soc. 2010;132:7881. doi: 10.1021/ja103041u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Stewart P, Chen M, Roush WR, Ess D. Org. Lett. 2011;13:1478. doi: 10.1021/ol2001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reviews of reactions of carbonyl compounds with crotylmetal reagents: Roush WR. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 2. Oxford: Pergamon Press; 1991. p. 1. Yamamoto Y, Asao N. Chem. Rev. 1993;93:2207. Denmark SE, Almstead NG. In: Modern Carbonyl Chemistry. Otera J, editor. Weinheim: Wiley-VCH; 2000. p. 299. Denmark SE, Fu J. Chem. Rev. 2003;103:2763. doi: 10.1021/cr020050h. Lachance H, Hall DG. Org. React. 2008;73:1..
- 6.Masamune S, Choy W, Petersen JS, Sita LR. Angew. Chem., Int. Ed. Engl. 1985;24:1. [Google Scholar]
- 7.(a) Stille JK. Angew. Chem. Int. Ed. Engl. 1986;25:508. [Google Scholar]; (b) Farina V, Krishnamurthy V, Scott WJ. Org. React. 1997:50. [Google Scholar]
- 8.(a) Ma S, Lu X, Li Z. J. Org. Chem. 1992;57:709. [Google Scholar]; (b) Buynak JD, Vogeti L, Chen H. Org. Lett. 2001;3:2953. doi: 10.1021/ol016142v. [DOI] [PubMed] [Google Scholar]
- 9.(a) Roush WR, Palkowitz AD, Palmer MJ. J. Org. Chem. 1987;52:316. [Google Scholar]; (b) Roush WR, Palkowitz AD, Ando K. J. Am. Chem. Soc. 1990;112:6348. [Google Scholar]
- 10.(a) Hattori K, Sajiki H, Hirota K. Tetrahedron Lett. 2000;41:5711. [Google Scholar]; (b) Hattori K, Sajiki H, Hirota K. Tetrahedron. 2001;57:2109. [Google Scholar]; (c) Ikawa T, Sajiki H, Hirota K. Tetrahedron. 2004;60:6189. [Google Scholar]; (d) Espeel PER, Piens K, Callewaert N, Van Der Eycken J. Synlett. 2008:2321. [Google Scholar]
- 11.Scheidt KA, Chen H, Follows BC, Chemler SR, Coffey DS, Roush WR. J. Org. Chem. 1998;63:6436. [Google Scholar]
- 12.Dess DB, Martin JC. J. Am. Chem. Soc. 1991;113:7277. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.