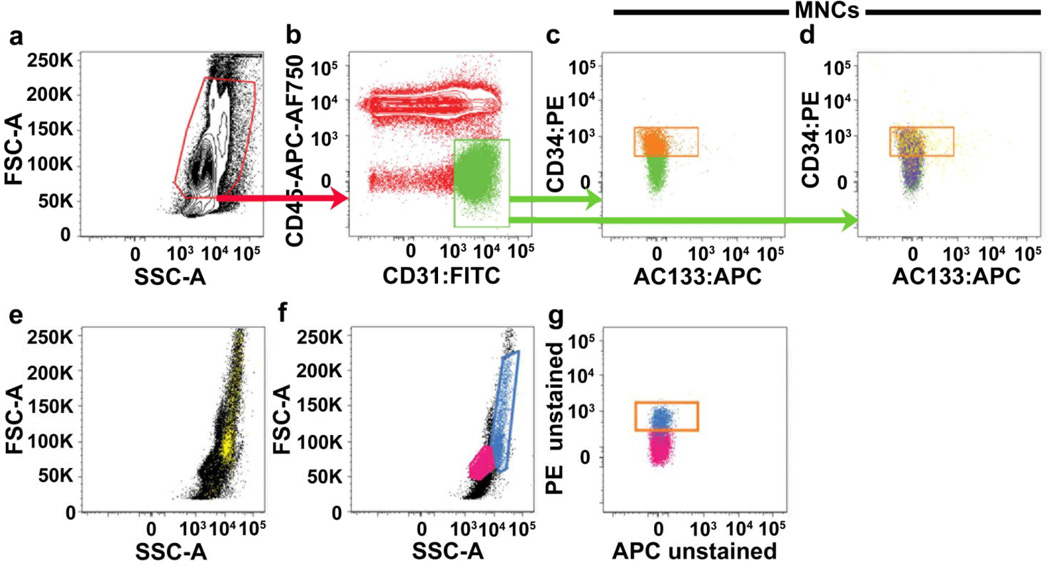
Figure 1. Threshold gating of mononuclear cells based on forward scatter and side scatter does not remove red blood cells, dead cell and monocyte contamination.
Representative PFC analysis of a CPT mononuclear cell preparation of peripheral blood either (a–e) stained with the six-antibody/viability marker panel (as defined in Methods), which includes glyA (red blood cell marker), CD14 (monocyte marker) and LIVE/DEAD® (cell viability marker) or (f–g) completely unstained. Mononuclear cells (red gate in a) are initially gated on a FSC/SSC plot in an attempt to exclude red blood cells, dead cells and debris, and sub-gated onto a bivariant antigen plot (b) for identification of CD31brightCD45− cells (green gate). CD31brightCD45− cells are further sub-gated to identify the CD34+AC133− subset (orange gate in c and d). In (d), glyA+ red blood cells and/or dead/apoptotic cells (purple events) and CD14+ monocytes (yellow events), contained within the initial mononuclear cell gate, are mapped onto the bi-variant plot and can clearly be seen distributed throughout the CD34+AC133− gate (orange gate). Monocytes are difficult to exclude based on their scatter profile. CD14+ monocytes (yellow cells mapped in e) can be seen distributed throughout the mononuclear cells when back-gated onto a forward scatter/side scatter plot (e). In analysis of unstained samples (f–g), cells that have the scatter profile of monocytes (blue gate in f and blue cells mapped onto g), are highly auto-fluorescent and contaminate the orange gate used for frequency analysis of CD34+AC133− cells. Lymphocytes are gated and mapped in pink for reference (f–g). Similar results were seen in 9 other samples from different donors.