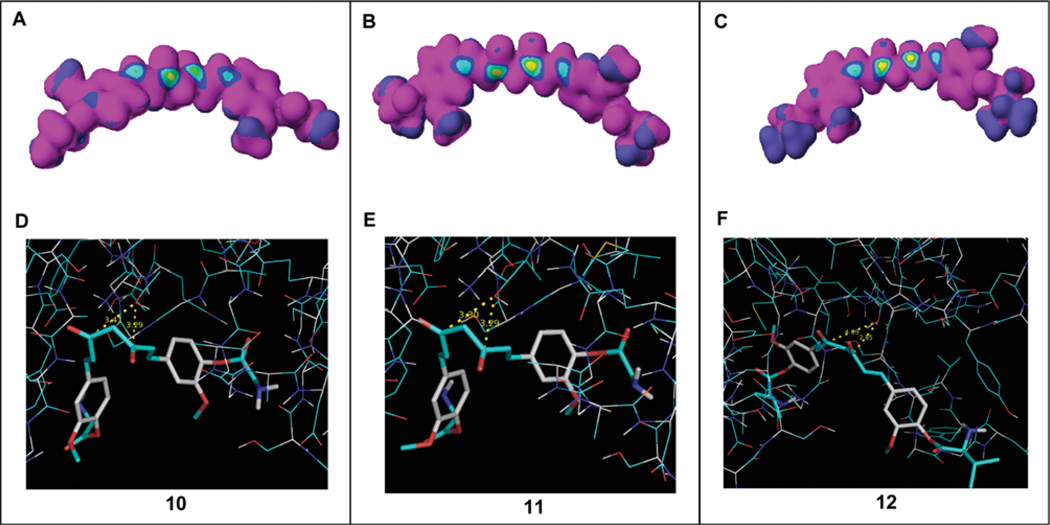
Figure 11.
Computational docking analysis. (A–C) Electron density was analyzed in curcumin analogs 10, 11 and 12 using CaChe software. The bull's eyes with a yellow center indicated high nucleophilic susceptibility. (D–F) These compounds were docked into the β5 subunit of the proteasome. The distances from the carbonyl carbons to the OH group of Thr1 of the β5 subunit were 3.29 or 3.41 Å in compound 10, 3.29 or 3.30 Å in compound 11 and 2.85 or 4.09 Å in compound 12. Compounds 10–12 are shown as stick models. The OH group in Thr1 is shown in red and white.