Table 2.
 | ||||||
|---|---|---|---|---|---|---|
| entry | substrate | R1 | R2a | conditionsb | product (R3) | yield (%) |
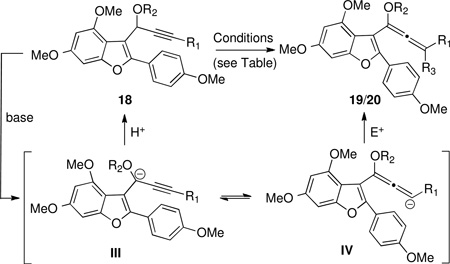
| 1 | 18a | Ph | Et | A, B, C, D, E, F | c | – |
| 2 | 18a | Ph | Et | G | 19a (SiMe3) | 40 |
| 3 | 18a | Ph | Et | H | 19b (SnBu3) | 95 |
| 4 | 18b | Ph | PMB | H | 19c (SnBu3) | 94 |
| 5 | 18c | Ph | OMB | I, J, K, L, M | c | – |
| 6 | 18d | H | PMB | N | 20 (H) | ~90 |
PMB=p-methoxybenzyl; OMB=o-methoxybenzyl
Conditions: A) KOt-Bu, MeOH, THF. B) MeLi, THF then MeOH. C) MeLi, THF then ZnCl2 then NH4Cl. D) t-BuLi, THF then imidazole. E) t-BuLi, THF then ZnCl2 then NH4Cl. F) Triton B®, DMSO, 50°C. G) 1.3 equiv. t-BuLi then TMSCI, THF, −30°C. H) t-BuLi, Et2O, −40°C then Bu3SnCl. I) t-BuLi, THF then MeOH. J) t-BuLi, TMEDA, THF then MeOH. K) t-BuLi, TMEDA, THF then t-BuOH. L) t-BuLi, TMEDA, THF then 2,6-di-t-butylphenol. M) t-BuLi, TMEDA, THF then imidazole. N) Triton B® (40% w/w MeOH), DMSO, 50°C
Propargylic ether was isolated unchanged.
