Abstract
Rhinolophidae, a family of echolocating bats, feature very baroque noseleaves that are assumed to shape their emission beam. Zhuang & Muller (Zhuang & Muller 2006 Phys. Rev. Lett. 97, 218701 (doi:10.1103/PhysRevLett.97.218701); Zhuang & Muller 2007 Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 76(Pt. 1), 051902 (doi:10.1103/PhysRevE.76.051902)) have proposed, based on finite element simulations, that the furrows present in the noseleaves of these bats act as resonance cavities. Using Rhinolophus rouxi as a model species, they reported that a resonance phenomenon causes the main beam to be elongated at a particular narrow frequency range. Virtually filling the furrows reduced the extent of the main lobe. However, the results of Zhuang & Muller are difficult to reconcile with the ecological background of R. rouxi. In this report, we replicate the study of Zhuang & Muller, and extend it in important ways: (i) we take the filtering of the moving pinnae into account, (ii) we use a model of the echolocation task faced by Rhinolophidae to estimate the effect of any alterations to the emission beam on the echolocation performance of the bat, and (iii) we validate our simulations using a physical mock-up of the morphology of R. rouxi. In contrast to Zhuang & Muller, we find the furrows to focus the emitted energy across the whole range of frequencies contained in the calls of R. rouxi (both in simulations and in measurements). Depending on the frequency, the focusing effect of the furrows has different consequences for the estimated echolocation performance. We argue that the furrows act to focus the beam in order to reduce the influence of clutter echoes.
Keywords: echolocation, Rhinolophidae, emission beam, furrows, morphology, sensory ecology
1. Introduction
Most Horseshoe bats (Rhinolophidae) have a prominent noseleaf featuring multiple furrows [1]. This family of bats emit calls containing predominantly a single-frequency component through their nostrils. At the onset and the offset of the calls, these bats typically also emit frequency-modulated components (see spectrogram in figure 1). However, the bandwidth of these components is limited in comparison with bats using frequency-modulated emissions.
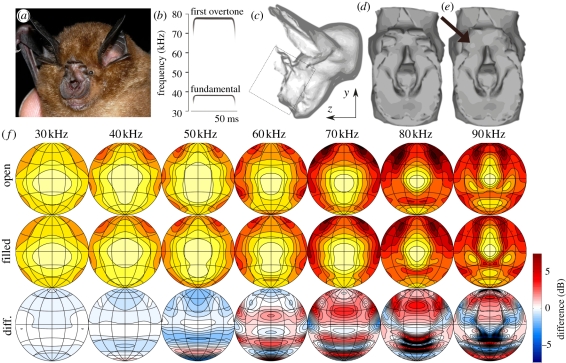
Figure 1.
(a) Picture of Rhinolophus sinicus, a species closely related to Rhinolophus rouxi, showing the complex noseleaf structure, including the furrows. Photo courtesy of Garreth Jones, Bristol University. (b) A stylized spectrogram of the call of R. rouxi. (c) Renderings of the three-dimensional model of the complete head used to simulate the spatial hearing sensitivity of R. rouxi. (d) Rendering of the detailed model of the facial structure of R. rouxi. (e) A version of the three-dimensional model in which the upper two rows of furrows were filled as was performed by Zhuang & Muller [3,4] (see arrow). (f) Upper row: the simulated far field emission patterns for the original facial morphology (i.e. model in (d)). Middle row: far field simulated patterns for the model in which the furrows were filled (i.e. model in (e)). Bottom row: difference between upper and middle row.
Given the co-occurrence of a specific type of call and facial morphology, it seems likely that the noseleaves of these animals have evolved to shape the emission beam at the narrow frequency bands used by these bats. Schnitzler & Grinnell [2] filled the furrows of Rhinolophus ferrumequinum with vaseline and recorded the beam before and after this manipulation. They found that filling the furrows disrupted the beam pattern at the constant frequency of this bat. However, Schnitzler & Grinnell [2] reported only on the effect of the furrows on the emission pattern for a single frequency. Moreover, these authors acknowledged that the alterations in noseleaf structure were not very precise and, so a detailed analysis further experiments are required.
More recently, such a detailed analysis based on acoustic simulations was performed by Zhuang & Muller [3,4] using a three-dimensional model of the facial morphology of Rhinolophus rouxi. They conclude that the emission beam pattern is largely unaffected by the furrows at the bat's constant frequency component. In contrast, at the lower end of the frequency-modulated component (i.e. at 60 kHz), the furrows are found to act as resonance cavities increasing the extent of the beam in elevation.
As the results of these two studies seem to contradict each other and as the results of the last, most detailed studies are somewhat unexpected for a number of ecological reasons (see §3), we propose to extend the analysis to find out whether the results remain valid. First, in addition to the noseleaf, we also include the effects of the pinnae into the analysis thereby removing an important limitation of the study by Zhuang & Muller [3,4] and Schnitzler & Grinnell [2]. Indeed, we have demonstrated earlier that the noseleaf effects on the emission beam are attenuated when considering the spatial sensitivity of the complete echolocation system [5]. Furthermore, for Rhinolophidae, including the effects of pinnae into the analysis is possibly even more important as Rhinolophidae use ear movements to generate localization cues [6]. Hence, the results we report take into account both the noseleaf and the (moving) pinnae morphologies. We use a recently developed information theoretic framework to estimate the effects of any changes in the sound field of R. rouxi on the information transfer rate during echolocation [6,7] for a range of frequencies. This framework allows to take into account the localization cues introduced by the moving pinnae.
2. Methods and results
As the methods employed in this paper have been discussed in detail elsewhere [6,7], we provide only a summary.
We constructed a detailed three-dimensional model of the complete head of R. rouxi. We also constructed a separate detailed model of the facial structure (figure 1). This was performed to allow modelling the structure of the noseleaf in greater detail (see Vanderelst et al. [6] for details).
The model of the complete head was used to numerically simulate the spatial hearing sensitivity of R. rouxi from 20 to 100 kHz. The model of the facial morphology was used to numerically simulate the emission beam. The emission beam was simulated both with open and filled furrows (figure 1). The dimensions of the furrows were measured on the model. The width of the furrows was about 2 mm. They were about 1 mm high and 1.3 mm deep (for more details about the sizes of the models, see electronic supplementary material, figure S4).
The resulting emission beam patterns for both versions of the noseleaf are plotted in figure 1 for different frequencies. From these plots, it can be seen that filling the furrows causes the main lobe to increase in size, as measured by its solid angle at −3 dB, for frequencies above 50 kHz. In figure 2b, the difference in focusing of the emission beam is plotted as a function of frequency. The difference between both models in focusing reaches a maximum between 60 and 75 kHz.
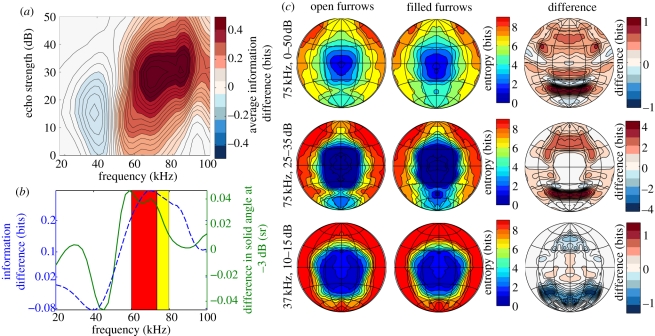
Figure 2.
(a) The difference in echolocation performance between the model with open and filled furrows as a function of frequency and echo strength. Positive numbers (red colours) indicate that the performance of the model with filled furrows is better than the model with open furrows. (b) The blue line denotes the difference in echolocation performance between the model with filled and open furrows averaged across echo strengths (positive values indicate that the performance of the model with filled furrows is better than the model with open furrows). The green line denotes the difference in solid angle at half power (i.e. −3 dB) in the emission beam of both models (positive numbers indicate the beam of the model with the filled furrows is larger than the beam of the model with open furrows). The red and yellow bands in panel (b) depict the range of the frequency-modulated component and the constant frequency component, respectively, as given by Neuweiler et al. [8] (c) The entropy, i.e. the uncertainty about the origin of an echo, as a function of azimuth and elevation for both models as well as the difference between both models. The first row shows the entropy averaged across the whole range of modelled echo strengths at 75 kHz. The second row depicts the results for 25–35 dB only at 75 kHz. The bottom row shows the results for 37 kHz and a limited range of echo strengths (10–15 dB). In the difference plots, positive values (red colours) indicate that the performance of the model with filled furrows is better than the model with open furrows.
The effect of the changes in the emission beam pattern on the localization performance was estimated using the model of echolocation in Rhinolophidae proposed by Vanderelst et al. [6]. This information theoretic model returns an estimate of the uncertainty (i.e. entropy) about the target direction from which an echo returns in number of bits. Note that a high uncertainty, i.e. entropy, corresponds with a low amount of information about the true position of the reflecting target giving rise to the echo. The model takes into account noise sources, echo strength and the effect of ear movements (see Vanderelst et al. [6] for implementation details and [7] for a theoretical grounding of the information theoretic model).
Figure 2a plots the change in localization performance owing to filling the furrows as a function of echo strength and frequency. From this plot, it can be seen that the effect of the furrows is largest in a frequency range that corresponds with the frequencies used by R. rouxi (including the frequency modulated (FM) part of the call). In this frequency range, the average amount of uncertainty about the origin of an echo, i.e. entropy, decreases (information increases) if the furrows are filled. An effect of the furrows can also be seen around the frequencies of the fundamental of R. rouxi (around 37 kHz). In this frequency range, the bat gains some information (entropy decreases) by having open furrows. These results (figure 2) also show that the effect of the furrows is not constant across echo strengths. Indeed, the effect of the furrows is largest for echo strengths around 30–35 dB.
Averaging, the difference in entropy across all modelled echo strengths (figure 2b), confirms that the effect of the furrows is largest in the range of frequencies contained in the first harmonic of R. rouxi. Moreover, this plot also shows the change in solid angle of the main lobe (at −3 dB) owing to filling the furrows as a function of frequency. This curve exhibits similar frequency-dependent behaviour as the localization performance indicating that filling the furrows results in the most pronounced beam size enlargements to fall in the range of frequencies contained in the first harmonic of R. rouxi (see also figure 1f).
The first row of figure 2c plots the entropy (low entropy values indicating low uncertainty about the origin of an echo, i.e. better localization performance) as a function of azimuth and elevation of target position at 75 kHz. Comparing the plots for the models with open and filled furrows reveals that the performance improvement associated with filling the furrows (figure 2a,b at 75 kHz) is mostly owing to a reduction of the height of the area in which echoes can be localized well for open furrows.
The first row of figure 2c considers the spatial distribution of target position uncertainty across echo strengths. However, we can also show the results for echo strengths in the range 25–35 dB only, i.e. the range for which the effect of the furrows is largest (figure 2a). Comparing again the results for both noseleaf models reveals a more pronounced contraction of the region of good localization performance. This decrease in size is again mainly owing to a reduction in height. However, some reduction in width can also be noticed in this range of echo strengths.
In contrast, at 37 kHz and 15–20 dB, the region of good localization is somewhat enlarged for open furrows when compared with filled furrows. The region of good localization is expanded towards lower elevations as demonstrated by the plots in the bottom row of figure 2c.
3. Discussion
The results of the study of Zhuang & Muller [3,4] are unexpected on ecological grounds for a number of reasons.
At the frequency of 60 kHz at which Zhuang & Muller [3,4] find effects of the noseleaf of R. rouxi, the emissions contain little energy. Therefore, it might be questioned whether the reported effects are functionally relevant to the bat. As, neither of the papers published by Zhuang & Muller [3,4] contain quantitative data about the changes in sound pressure caused by filling the furrows it is difficult to interpret the possible relevance of the presented changes to the emission beam pattern.
Zhuang & Muller [3,4] argue that the adaptive value of an elongated beam at 60 kHz is that it enables the bat to track its height above the ground. However, in their analysis of the effect of the furrows on the main lobe, the main lobe is elongated towards higher elevations by the furrows. One would expect adaptations to the beam intended to track the ground to be found at the lower boundary of the beam. Indeed, the beam of Eptesicus fuscus has been found to contain a second main lobe aimed to the ground that has been conjectured to serve this very role [9]. Moreover, this functional interpretation raises the question why no bats using frequency-modulated calls with furrows in their noseleaves have been identified.
In contrast to Zhuang & Muller [3,4], we find that the furrows act to decrease the size of the main lobe resulting in increased focusing and directivity for the complete frequency range of the bat's calls. The reduction in main lobe size has an effect on the predicted localization performance in the same frequency range. The area in which R. rouxi is predicted to be able to locate targets well is smaller with the (open) furrows than with the filled furrows. This causes overall localization performance to decrease.
While it might seem odd at first that any morphological feature would decrease the localization ability, this result ties in with other reports on the effects of noseleaves in bats. In Micronycteris microtis [7], the noseleaf similarly acts to reduce the area in which the bat can locate targets by focusing the emission beam. Monte Carlo simulations showed that the focused beam reduces the influence of clutter [5]. Indeed, we have previously argued that noseleaves are mostly instruments that serve clutter rejection. In this light, it is unsurprising that R. rouxi, which typically hunts in highly cluttered environments, would have evolved a mechanism to further sharpen its emission beam and reduce the influence of unwanted clutter echoes.
In sum, based on simulations and measurements on a physical mock-up of the facial morphology of R. rouxi (see electronic supplementary material), we tentatively suggest that the furrows of R. rouxi help focusing the emission beam increasing clutter rejection for the complete frequency range employed by the bat. Because of the small frequency range employed by Rhinolophidae, we conjecture that furrows might be a more efficient way of focusing the beam than having a longer, plainly shaped, noseleaf without furrows. Possibly, the same mechanism as proposed by Zhuang & Muller [3,4], i.e. the furrows acting as resonant cavities, allows such focusing to take place. Therefore, we propose that the noseleaf with furrows, might be the specialized (in terms of frequency range) counterpart of the lancet shaped noseleaf featured by broadband bats, such as Phyllostomus discolor and M. microtis [5].
The results of Zhuang & Muller [3,4], and the results presented here are in obvious disagreement. As both analyses are based on simulation studies, an experimental re-evaluation of the effects of the furrows on the emission beam pattern of Rhinolophidae seems the most promising approach to resolve this inconsistency. Using current state-of-the-art multi-channel recording devices, it should be possible to measure the emission pattern of Rhinolophidae with filled and open furrows. Measuring the emission pattern on a life bat instead of a physical mock-up has the advantage that all tissue characteristics are taken into account. Indeed, while there is little direct evidence that the properties of the tissue around the nostrils and of the noseleaf influence the emission beam pattern of bats, it has been suggested that these could be of importance [10].
References
- 1.Nowak R. M., Walker E. P. 1994. Walker's bats of the world. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 2.Schnitzler H. U., Grinnell A. 1977. Directional sensitivity of echolocation in the horseshoe bat, Rhinolophus ferrumequinum I. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 116, 51–61 10.1007/BF00605516 (doi:10.1007/BF00605516) [DOI] [Google Scholar]
- 3.Zhuang Q., Muller R. 2006. Noseleaf furrows in a horseshoe bat act as resonance cavities shaping the biosonar beam. Phys. Rev. Lett. 97, 218701. 10.1103/PhysRevLett.97.218701 (doi:10.1103/PhysRevLett.97.218701) [DOI] [PubMed] [Google Scholar]
- 4.Zhuang Q., Muller R. 2007. Numerical study of the effect of the noseleaf on biosonar beamforming in a horseshoe bat. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 76(Pt. 1), 051902 10.1103/PhysRevE.76.051902 (doi:10.1103/PhysRevE.76.051902) [DOI] [PubMed] [Google Scholar]
- 5.Vanderelst D., De Mey F., Peremans H., Geipel I., Kalko E., Firzlaff U. 2010. What noseleaves do for FM bats depends on their degree of sensorial specialization. PLoS ONE 08, e11893 10.1371/journal.pone.0011893 (doi:10.1371/journal.pone.0011893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vanderelst D., Reijniers J., Steckel J., Peremans H. 2011. Information generated by the moving pinnae of Rhinolophus rouxi: tuning of morphology at different harmonics. PLoS ONE 1, e20627 10.1371/journal.pone.0020627 (doi:10.1371/journal.pone.0020627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reijniers J., Vanderelst D., Peremans H. 2010. Morphology-induced information transfer in bat sonar. Phys. Rev. Lett. 105, 148701. 10.1103/PhysRevLett.105.148701 (doi:10.1103/PhysRevLett.105.148701) [DOI] [PubMed] [Google Scholar]
- 8.Neuweiler G., Metzner W., Heilmann U., Rubsamen R., Eckrich M., Costa H. 1987. Foraging behaviour and echolocation in the rufous horseshoe bat (Rhinolophus rouxi) of Sri Lanka. Behav. Ecol. Sociobiol. 20, 53–67 10.1007/BF00292166 (doi:10.1007/BF00292166) [DOI] [Google Scholar]
- 9.Ghose K., Moss C.F., Horiuchi T.K. 2007. Flying big brown bats emit a beam with two lobes in the vertical plane. J. Acoust. Soc. Am. 122, 3717–3724 10.1121/1.2799491 (doi:10.1121/1.2799491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuc R. 2010. Morphology suggests noseleaf and pinnae cooperate to enhance bat echolocation. J. Acoust. Soc. Am. 128, 3190–3199 10.1121/1.3488304 (doi:10.1121/1.3488304) [DOI] [PubMed] [Google Scholar]