Fig. 3.
Validation of putative activated state against experimental data. (A) Changes in solvent accessibility of TMD residues upon channel activation, either from substituted cysteine modification rates by Ag+ (23, 24) or from normal mode analysis (NMA). Blue bars are ln (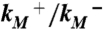 ), where
), where 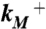 and
and 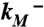 are modification rate constants in the presence and absence of ATP. Red bars are differences in solvent accessible surface area (ΔSASA, in Å2 and shown as scaled by a factor of 2) of residues between the activated and resting states. ΔSASA was calculated by mutating each residue to cysteine in the two states and taking the difference in accessible surface area for the sidechain only. Given the sequence differences between zfP2X4R and rP2X2R, it is perhaps not very meaningful to quantify the comparison. Nevertheless, after excluding L340, the correlation between the experimental and calculation data has an R2 of 0.58. Note that a change in the motional amplitude used for generating the activated model changes the magnitudes of ΔSASAs for the individual residues but not their signs and rankings, so the correlation with experimental data is preserved. (B) The lateral fenestration in the resting and activated states. D61, Q329, and I336 are shown as spheres in darker colors, which are visible only when the spheres are exposed.
are modification rate constants in the presence and absence of ATP. Red bars are differences in solvent accessible surface area (ΔSASA, in Å2 and shown as scaled by a factor of 2) of residues between the activated and resting states. ΔSASA was calculated by mutating each residue to cysteine in the two states and taking the difference in accessible surface area for the sidechain only. Given the sequence differences between zfP2X4R and rP2X2R, it is perhaps not very meaningful to quantify the comparison. Nevertheless, after excluding L340, the correlation between the experimental and calculation data has an R2 of 0.58. Note that a change in the motional amplitude used for generating the activated model changes the magnitudes of ΔSASAs for the individual residues but not their signs and rankings, so the correlation with experimental data is preserved. (B) The lateral fenestration in the resting and activated states. D61, Q329, and I336 are shown as spheres in darker colors, which are visible only when the spheres are exposed.

