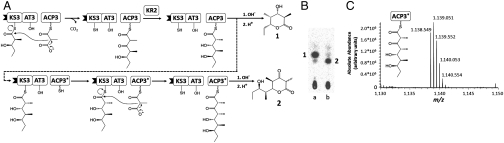
Fig. 7.
An engineered chimeric ACP exhibits iterative chain elongation. (A) Schematic representation of the reactions catalyzed by [KS3][AT3] with the wild-type ACP3 (23) and the engineered ACP3 (SHIV78, shown as ACP3*). The reaction involving wild-type ACP3 follows the solid arrows leading to the formation of compound 1 (a triketide ketolactone). The reaction involving ACP3* is predicted to follow the dashed arrow, leading to the formation of compound 2 (a tetraketide ketolactone). (B) Radio thin-layer chromatography for the reaction of [KS3][AT3] with wild-type ACP3 (lane a) and ACP3* (lane b). Compounds 1 and 2 are labeled, and have identical Rf values to those of authentic standards (31) (C) The identity of the tetraketide was confirmed by an established proteolytic LC-high-resolution mass spectroscopy (HRMS) method (24). The HRMS trace (integrated from 60–63 min of the elution gradient) for the ACP3* reaction is shown. The ACP fragment shown in the panel (peptide sequence AFSELGLDS*LNAMALR, where the phosphopantetheine moiety containing the tetraketide thioester is covalently bound to the active serine, S*) has a calculated monoisotopic m/z of 1,138.551 (z = +2). Additional fragment isotopes are labeled and follow the expected isotopic distribution. The precision of the HRMS device is 2 millimass unit.