Abstract
The oceanographic conditions in the north Pacific have shifted to a colder period, Pacific sardine (Sardinops sagax) biomass has declined precipitously in the California Current, the international sardine fishery is collapsing, and mackerel (Trachurus symmetricus and Scomber japonicus) are thriving. This situation occurred in the mid-1900s, but indices of current oceanographic conditions and the results of our acoustic-trawl surveys indicate it likely is recurring now, perhaps with similar socioeconomic and ecological consequences. Also alarming is the repetition of the fishery's response to a declining sardine stock—progressively higher exploitation rates targeting the oldest, largest, and most fecund fish. Furthermore, our data indicate the recent reproductive condition of sardine is poor, and their productivity is below modeled estimates used to derive the current fishery-exploitation rates. Consequently, the sardine population has been reduced to two cohorts that are unlikely to produce an appreciable new cohort. Thus, a near-term recovery of this important stock is unlikely, depending on the return of warmer oceanographic conditions, reduced pressure from mackerel species, and perhaps the adoption of a more precautionary strategy for managing the residual sardine population.
Keywords: Pacific Decadal Oscillation, upwelling system, pelagic community, management
It is widely recognized that many fish stocks worldwide have collapsed because of overexploitation (1), but other stocks wax and wane, perhaps because of cyclical environmental factors (2), anthropogenic factors, or both (3). A paradigmatic example of periodic fish abundance and exploitation is the Pacific sardine (Sardinops sagax) fishery in the northeast Pacific (Fig. 1). Fossil evidence from the last 1,700 y indicates that the stock abundance is cyclical with a period of ∼60 y, independent of fishing (4).
Fig. 1.
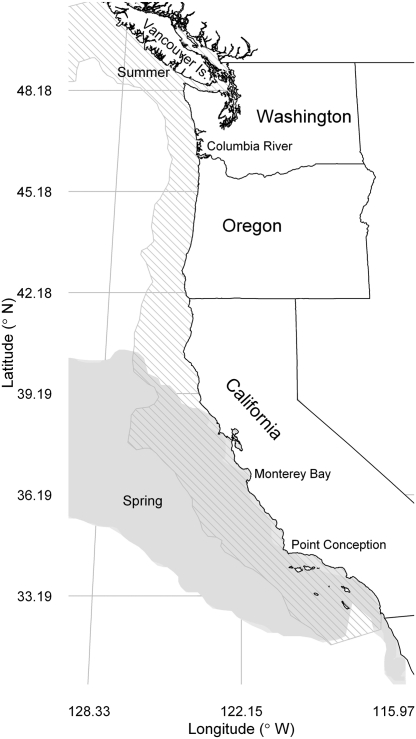
Potential sardine habitat during spring (April) and summer (July) predicted using satellite-sensed oceanographic conditions during 1998–2009 (9). In agreement with our model predictions (9, 10), Pacific sardine (Sardinops sagax) spawn offshore of California during spring and then migrate north to feed in the coastal upwelling regions in summer (10, 13). The oldest, largest, fattest, most fecund sardine migrate farthest north (41).
Because sardine and other small pelagic fishes comprise the majority of landings worldwide, and these stocks exhibit large interannual and interdecadal variations of abundance and collapse (5), long-term ecological studies are conducted to understand better the relationships of pelagic fishes, their environment, and the fisheries. Notably, the California Cooperative Oceanic Fisheries Investigations (CalCOFI) began multidisciplinary time series in 1949 to study the ecological aspects of the sardine population growth and collapse off the west coast of the United States in the early to mid-1900s (6). During this period, the “northern” sardine stock (7) and the international sardine fishery burgeoned, spanning from Mexico to Canada; then, because of overfishing during periods of low productivity (8), it contracted abruptly and ultimately halted, with significant socioeconomic effects, for nearly 20 y (6).
Here, we examine the many parallels between the growth and collapse of the northern sardine stock in the early to mid-1900s and the current situation. We begin with a review of the scientific literature to glean a number of characteristics of this historical stock and its environment. Then, using a combination of references and our own data and analyses (9–11), we systematically show that all these characteristics are present again.
Results
A Fish Story (1930s–1950s).
This first account is a synthesis of the literature aimed at identifying characteristics of the collapse of the sardine stock and fishery in the last century. In the 1930s and 1940s, sardine dominated the fish landings in North America (6) and comprised the largest single-species fishery in the Western hemisphere (12). The stock migrated seasonally, spawned principally in the spring offshore southern and central California, and foraged in the summer in the coastal upwelling regions off northern California, Oregon, Washington, and Vancouver Island (Fig. 1) (9, 13). The mostly unregulated fishery intensely targeted the larger migrating sardine in the northern upwelling regions (6, 8). Only 5 y after the landings peaked there during the 1943–1944 season (6), the population did not appear off Vancouver Island. The following season, no sardine were found north of California. By 1952, the sardine fishery north of Monterey Bay had ended (6).
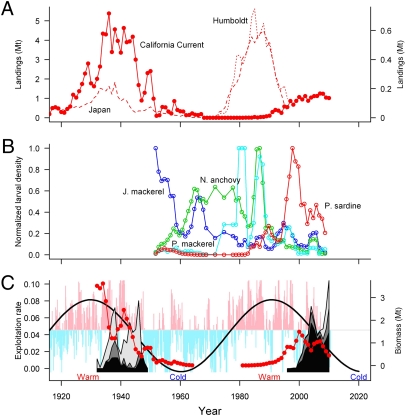
A few years earlier, apparently forecasting the collapse of the sardine fishery off the west coast of the United States, nearly identical events occurred in a sardine fishery on the western side of the Pacific basin. Landings off Japan peaked in 1938, 2 y after the landings peaked off the United States and Canada, and dropped to a historical low in 1945, approximately 10 y before the United States sardine fishery collapsed (Fig. 2A) (12). This coincidence prompted theories of basin-scale oceanographic forcing (14).
Fig. 2.
(A) Landings in Mt of the sardine fisheries off the west coasts of North America (solid line, right y-axis), Chile (dotted line, left y-axis), and Japan (dashed line, left y-axis) (12). (B) Normalized 3-y-running mean larval densities of northern anchovies (green line), Pacific sardine (red line), Pacific mackerel (aqua line), and jack mackerel (blue line) off southern California indicate changes in the dominant pelagic fish species in the CCE: sardine in 1990–2010, jack mackerel in the 1950s, anchovy in the 1960s–1980s, and Pacific mackerel in the 1980s. (C) Biomass of the northern sardine stock age 2 y+ in the CCE (25) (red); cumulative exploitation rates (catch divided by estimated abundance) from the fisheries off Oregon (black), Washington (dark gray), and Vancouver Island (light gray); and monthly PDO indices (vertical blue and pink bars) oscillating with a 60-y period; Our fit of a 60-y cycle to the monthly PDO index (black line) predicts the indices will be maximally negative in 2020 and suggests another warm period conducive to sardine in 2035. The dramatic drop in the exploitation rate of the northern fisheries in the 1940s is the result of the decline and interruption of the feeding migration that occurred 5 y after the landings peaked in the northern fisheries. In 2010, the exploitation rate in the northern fisheries peaked at a new maximum. The time series of sardine landings used to construct A were obtained from refs. 12 and 25. The time series of larval densities of northern anchovies, Pacific sardine, Pacific mackerel, and jack mackerel in B are from the 1951–2010 CalCOFI surveys (http://calcofi.org).
During the period of declining and then low sardine biomass in the northeast Pacific, jack mackerel (Trachurus symmetricus) thrived and dominated the biomass of pelagic fishes, followed by northern anchovy (Engraulis mordax) and then Pacific mackerel (Scomber japonicus) (15) (Fig. 2B). This succession led to a pivotal hypothesis that warm periods (e.g., 1927–1947) favor sardine in this region, and cold periods (e.g., 1948–1977) favor anchovy (16, 17). It also appears that the periods of gradual transition favor mackerel (15). In 1978, the Pacific Decadal Oscillation index (PDO) (18) described another cold-to-warm transition (16), and the northern sardine stock began to increase again off the west coast of the United States (Fig. 2 B and C) (19).
Summarizing more than 60 y of retrospective analyses of these and other data, the collapse of the sardine fishery in northeast Pacific was characterized by
1. Negative phase of the PDO and decline in the Japanese sardine stock (basin-scale concordance);
2. Focus of the fishery on the oldest, largest, most fecund fish (fishery focus);
3. Decline in the sardine biomass below a critical level (critical biomass);
4. Shift in the dominant species and their schooling behavior (species alternation); and
5. Halt in the seasonal sardine migration (seasonal migration).
Each of these signs is elaborated in the next five sections.
Basin-scale concordance.
There is compelling evidence that low-frequency oceanographic fluctuations triggered collapses of the sardine populations in both margins of the north Pacific in the mid-1900s (Fig. 2A) (12, 15). The PDO identifies “cold” and “warm” periods in the north Pacific, alternating every 20–30 y (18). A 21-y warm period from 1925–1946 favoring sardine transitioned to a 29-y cold period from 1947–1976. Then, in the 1980s and 1990s, another warm period appears to have promoted increases in the sardine biomasses off Japan and Chile (12) and off the United States and Canada (Fig. 2A) (12, 16). In addition to the effects of the changing environment, the collapse of the northern sardine stock in the California Current ecosystem (CCE) was attributed in part to the international fishery (6, 8).
Fishery focus.
The oldest, largest, and most fecund sardine in this stock complete a seasonal migration from their spawning area offshore of southern California in the spring to their coastal feeding grounds off Oregon, Washington, and Vancouver Island in the summer (Fig. 1) (13). Before the collapse of this sardine population, these old fish, selected for their large size and high fat content, were intensely and increasingly targeted in the northern fisheries (Fig. 2C), stripping the population of its ability to reproduce and recruit successfully (6, 8). The sardine fishery was not managed, except for a limit on the fraction of the catch used for “reduction” (fish meal and oil products), and landings were governed solely by socioeconomic considerations. Population exploitation rates, exceeding 20% per year when the ocean temperatures were below average and sardine production was low, resulted in a decline in the sardine biomass (19). The northern fisheries increasingly exploited the large migrating fish until they were depleted locally (Fig. 2C), and the fate of this sardine population was determined after just two seasons with unsuccessful spawning (8). Exploitation of the declining stock continued off California until a moratorium on their landings was imposed in 1967. By then, however, the sardine stock had virtually disappeared (6).
Critical biomass.
When the total biomass of age 2-y-plus individuals, comprising most of the spawning stock biomass, decreased below 0.74 million tons (Mt) in 1948 (Fig. 2C) (20), and most of the largest individuals had been removed by the fishery, sardine progressively disappeared from the fisheries off Canada and then off the northwest United States (6). Thus, a sardine population below this critical biomass, in combination with unfavorable environmental conditions indicated by negative and declining PDO values (Fig. 2C), precipitated the collapse by preventing the remaining sardine from reproducing successfully (8). Through intense, localized harvesting, the fishery reduced the number of age classes and consequently the behavioral diversity of the stock, reducing its ability to adapt readily to unstable environmental conditions (8).
Species alternation.
In addition to identifying oceanographic and meteorological conditions favoring sardine, the PDO also may explain the sequential dominance of multiple species of small pelagic fishes in upwelling ecosystems throughout the Pacific (Fig. 2B) (16). The warm and cold periods, each lasting approximately 3 decades (Fig. 2C), alternately favor sardine and anchovies, respectively (Fig. 2B) (15, 16). During the shorter periods of gradual transition, e.g., in the 1950s and the 1980s, jack and Pacific mackerel populations grow rapidly and thrive (Fig. 2B) (15). During the last cold period, 1947–1976 (29 y), when sardine abundances were low, few sardine were found in single-species schools; rather, they schooled within the more abundant Pacific and jack mackerel schools (19). This “school trap” behavior may have a negative dampening effect on sardine recruitment and growth if it reduces their ability to search for optimal feeding and reproductive conditions (21), and their eggs and larvae may become forage for the other coaggregating fish species (22).
Seasonal migration.
In the mid-1900s, a conspicuous indicator of the collapse of the northern sardine stock in the CCE was the interruption of the seasonal feeding migration led by older, larger individuals. Sardine migrate seasonally when their energetic gains exceed the costs of swimming to and foraging in upwelling areas with higher primary productivity (Fig. 1) (23). Thus, sardine typically do not begin to migrate from the area in which they were spawned and recruited until they are more than 1 y old and longer than about 20 cm. When most of the larger fish were depleted by the fishery, the Canadian fishery abruptly declined in the mid-1940s, and the migration soon stopped (6). By 1949, the sardine stock did not migrate to Vancouver Island, and during 1950 the stock did not migrate north of California (6). During the late 1940s and early 1950s, the sardine fishery continued off California. During this time, the remaining sardine presumably experienced decreased foraging opportunities, their physiological condition deteriorated, and the population failed to produce substantive recruitments (8). Consequently, the sardine population plummeted in the early 1950s.
A Fish Story (1980s–Present).
This second account is both a synthesis of the literature and our analysis of results from our acoustic-trawl surveys (surveys that combine echosounder and net sampling) of multiple coastal pelagic fish species off the West coast of the United States during spring 2006, spring and summer 2008, spring 2010, and spring 2011 (10, 11). We systematically evaluate the characteristics of the last collapse to foresee if the sardine population and fishery off the west coast of North America is likely to collapse again.
Basin-scale concordance.
The Chilean sardine stock peaked in 1985 and then declined steeply (12), and Japanese sardine catches peaked in 1988 and then also dropped sharply (Fig. 2A) (12). These collapses were coincident with a declining period of the PDO in the 1990s (Fig. 2C). Meanwhile, off the west coast of the United States, Pacific mackerel were abundant between 1980 and 1998 (18 y) (Fig. 2B) (24). Because of competition from Pacific mackerel and the potential dampening effect on recruitment related to mixed-species aggregations (17, 21), the peak in the population size of the northern sardine stock in the CCE lagged the peaks in the northwest and southeast Pacific by approximately a decade (Fig. 2A) (15).
Based on a retrospective analysis of CalCOFI and other data, the sardine abundance in the CCE was predicted to peak around 1998 (15). It peaked in 2001 (Fig. 2C and see Fig. 4). Because of a strong 2003 cohort (Fig. 2C) (25), the northern sardine stock in the CCE increased again, peaked in 2006, and since then has been declining precipitously (Fig. 2C and see Fig. 4). Catches of pink, chum, and sockeye salmon, which historically track the rise and fall of the sardine populations in the Pacific basin (26), peaked with the PDO, around 1990 (27).
Fig. 4.
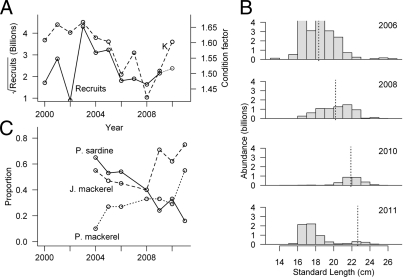
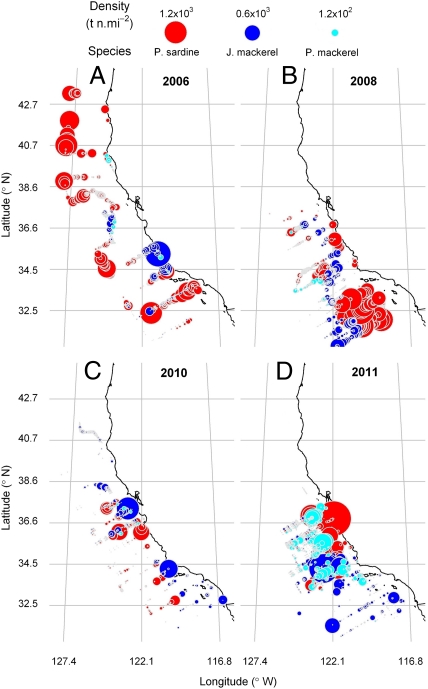
(A) Estimates of sardine recruits (28) and associated condition factors (K) (34) from our analysis of sardine landings off Oregon and Washington. Since 2003, the decrease in K correlates with the decline in sardine production. (B) Biomass-weighted length distributions of the northern sardine stock from our spring acoustic-trawl surveys (11). The mean lengths (dashed line) of the 2003 cohort (perhaps including 2003- and 2004-y classes) indicate that its biomass has diminished greatly between 2006 and 2011. The mean length of the most recent cohort (perhaps including 2009- and 2010-y classes) is small, ∼17 cm. The number of recruits (in billions; solid line) is measured on the left y-axis. (C) Proportions of trawl catches in our surveys (11, 12) that included sardine and mackerel. The trends indicate that catches of only sardine (indicating monospecific schools) are decreasing, and catches of jack and Pacific mackerel are increasing during spring surveys off California. These trends indicate that sardine are schooling increasingly with mackerels. Note: in A, the 2010 recruitment was estimated as the abundance of the smaller modal class of sardine, in the 2011 acoustic-trawl survey (shown in B). Recruitment indices used in A are from ref. 25. Condition factor used in A: data courtesy of the Oregon and Washington Departments of Fish and Wildlife.
Fishery focus.
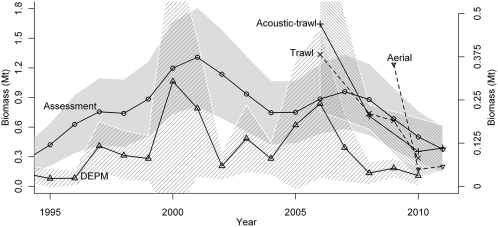
During the recent exploitation period, sardine landings by the United States and Canadian fisheries commonly are perceived as being low relative to those during the mid-1900s (Fig. 2A), partly because of the existence of harvesting control regulations (25). Although the United States and Canada have used separate management policies, they are linked by the proportions of the sardine stock available for fishing in each season and region, set in the annual harvest guidelines, derived from the United States assessment model (28). Until recently, Canadian managers assumed that 10% of the northern sardine stock estimated by the United States assessment migrated into Canadian waters during summer (29). Since 2009, however, based on the results of Canadian trawl surveys and despite the steady decline of all fishery-independent estimates of sardine abundance (Fig. 3), the Canadian management increased its estimate of the migrating proportion from 10 to 18%, effectively increasing allowable catches there by ∼80% (29).
Fig. 3.
Estimates of spawning stock biomass with 95% confidence intervals (shaded areas) for the northern sardine stock off North America from assessment (open circles; ref. 25), daily egg production method (DEPM) (open triangles; ref. 39), and our acoustic-trawl methods (x symbols and solid line, ref. 11), and estimates of abundance (95% confidence intervals were omitted for clarity) off southern Vancouver Island from trawl surveys (x symbols and dashed line; ref. 28) and off Oregon and Washington from aerial surveys (small triangles and dashed line; ref. 25). The y axis on the right refers to the trawl-survey estimates. Some estimates are of the entire stock (continuous lines), and some are of unknown proportions of the stock (dashed lines).
Under the environmental conditions and management constraints on the fishery, the northern sardine stock recovered in the 1990s to less than one-third of the biomass of the 1930s. It started to decline in 2001 and in 2010 reached the lowest spawning stock biomass values since the beginning of Federal management (Fig. 3) (25). The exploitation rates of migrating sardine in the northern fisheries off Oregon, Washington, and Vancouver Island started to rise in the late 1990s and then temporarily decreased because of a strong recruitment from the 2003 cohort (Fig. 2C). However, in the absence of another substantial recruitment, the exploitation rates of the aging sardine stock have increased dramatically since 2006 (Fig. 2C) (25). Thus, the northern fisheries again are increasingly targeting the migrating sardine, which are the largest, oldest, and most fecund animals. In 2010, the total population exploitation rate peaked at ∼23% (25), exceeding the high rates conducive to the recession of the sardine stock in the 1940s, when sardine productivity was declining (8).
Critical biomass.
The latest extended warm period of the PDO, initiated in 1977 (18, 30), created conditions considered beneficial for sardine, such as improved inshore retention of early life stages, increased stability of the water column, and adequate plankton communities for forage (31). Nevertheless, the northern sardine stock did not recover in the CCE until circa 1990, perhaps when the hypothesized dampening effect of Pacific mackerel subsided (Fig. 2C). In subsequent years, boosted by warm, episodic El Niño events, sardine biomass increased, exceeding the spawning stock biomass of 0.76 Mt in 1997 (Fig. 3), when sardine again were found extensively off Vancouver Island (32). This event marked the expansion of their seasonal migration to the historical northern limit. Throughout their geographical range, sardine growth was rapid, recruits per unit spawning stock biomass were high (25), and there were multiple successful recruitment events (32). The fishery was fully reestablished, but, because of the lower population size, management constraints, and no reduction fishery, it yielded maximum catches that were only about one-quarter of the historical values (Fig. 2A) (6, 25). Nevertheless, since 2006 (2007 in the assessment time series), the spawning stock biomass indices have declined steadily (Fig. 3).
The current decreasing trend of biomass of the northern sardine stock in the CCE is witnessed by all fisheries-independent estimates of abundance (Fig. 3) (11, 25) and can be related to the beginning of an unstable period of the PDO and the fishery focus (Fig. 2C). In 2010, all spawning stock biomass estimates were lower than the 0.74 Mt estimated for sardine in 1948 (20) when the stock failed to migrate to Vancouver Island after being severely depleted of the older age classes (6). If sardine migrate in summer to the highly productive upwelling regions off Oregon, Washington, and Vancouver Island, they will be targeted increasingly by the United States and Canadian fisheries. If sardine do not migrate to their summer feeding grounds, they may not gain sufficient weight for extensive spawning during the following spring (33).
Based on market-sampling data, a notable trend associated with the current decline in sardine biomass is the gradual loss of fitness of the adult population, evidenced by a general decrease of their condition factor (K) (34) before the spawning season (Table S1). A progressive decrease in their condition, suppressing their reproductive potential, explains their low recruitments from 2004–2008 (Fig. 4 A and B). Improved sardine condition during 2009–2010 corresponded to a slight increase in recruitment. A modest recruitment in 2011 (Fig. 4B), perhaps comprising both the 2009- and 2010-y classes, was observed in the results of the latest acoustic-trawl survey conducted in April 2011 (Fig. 5D). However, because these new recruits may not find it energetically feasible to migrate to the feeding grounds until their second year of their life (23), they may not gather and store enough energy to reproduce extensively, curtailing future recruitment.
Fig. 5.
Distribution and abundance of sardine and mackerel off the west coast of the United States during springs of 2006, 2008, 2010, and 2011, estimated from our acoustic-trawl surveys (11). Our survey results show that in 2011 sardine were surpassed by jack and Pacific mackerel as the dominant epipelagic fish species.
Species alternation.
In addition to the effects of the environment and fisheries, the growth and reproduction of the 2011 recruitment may be affected by both competition and predation. Our survey data show that jack mackerel has been abundant in the sardine habitat in the CCE since at least 2006 (Fig. 4C), and the Pacific mackerel biomass increased dramatically in 2011 (Fig. 5D). Our analysis also shows that these recent increases in the abundance of mackerel are coupled with a significant reduction of monospecific sardine schools in the CCE since 2004 (Fig. 4C). Schooling with mackerel could affect sardine condition, because mackerel tend not to migrate as far north or as near shore as sardine, presumably preferring different environments (24) and prey. Mackerel may forage on small sardine and sardine eggs and larvae if the species coaggregate during sardine spawning (22).
Seasonal migration.
Currently the likelihood for a renewed cessation of the seasonal sardine migration is high. Our survey results show clearly that the last significant year-class was spawned by the northern stock in the CCE in 2003 (Fig. 4A); this year-class peaked in biomass in 2006 (Fig. 3) and since then has been depleted severely by natural and fishery-related mortality (Figs. 2C and 4B). The April 2011 survey results (Fig. 5D) indicate that the large 2003 cohort, which peaked in abundance in 2006, has been greatly surpassed in numbers by the modest number of recruits observed in 2011 (Fig. 4B). The biomass of the potentially migrating fish [i.e., the largest individuals with lengths in excess of 20 cm (13)] is well below the past critical value of 0.74 Mt of age 2-y-plus sardine, and the residual large fish are increasingly mixed with mackerel. These signs and the associated potential negative-feedback mechanisms (17, 21) indicate the sardine stock is collapsing and that there will be long-lasting ecological and socioeconomic effects if history is repeating.
Discussion
The recent reduction in sardine biomass has been accompanied by a decrease in per-capita reproductive output. This recruitment depensation (35) appears to occur when individuals of the decreasing population are unable to exploit their environment for optimal foraging and reproduction. Theoretically, when minority species school with other species of fish, their adult populations are fragmented, migration routes are disrupted, and foraging and therefore reproductive potentials are reduced (33). However, the effects of depensation are difficult to identify and quantify (36), and population models frequently assume, on the contrary, that fish increase their reproduction in response to a decrease in population size (37). Therefore, assessment models likely overestimate reproductive success at low population sizes (37). Consequently, management inadvertently can allow overharvesting, which may cause subsequent stock recovery to be slow and incomplete (33, 35, 36, 38).
In the 1940s, the larger, migrating sardine were targeted aggressively, the number of cohorts declined (8), and their seasonal migration stopped (6). These factors likely reduced the duration of spawning events and the stock's ability to thrive in an unstable environment (8). The harvesting pressure on the residual sardine stock continued off southern California until the early 1960s (6). Consequently, when the CCE experienced another warm period in the 1980s, the recovery of the northern sardine stock in the CCE apparently was delayed by a decade (15) (Fig. 2A). By that time, the warm period had begun to wane, and the stock grew to only about one-third of its historical size and remained large for only half as long before declining again in association with the onset of another cold period (Fig. 2C).
Currently, the sardine population appears to be comprised almost entirely of two cohorts. Our survey results show that the older cohort now is almost negligible, and the recent cohort is small. Nearly gone are the largest fish with high reproductive potential, which spawn more frequently, during longer periods, and with the largest egg batches (39). Our analysis of landings data shows that the residual large fish are exploited increasingly (Fig. 2C). Consequently, the reproductive potential of the population is greatly diminished. This effect likely will increase recovery times relative to a population with similar biomass but with more cohorts and diverse behaviors (8, 33).
In 2011, based on the results of our acoustic-trawl survey, the sardine biomass of the northern stock in the CCE no longer dominated that of other epipelagic fish species, and sardine were found increasingly in mixed-species schools (Figs. 4C and 5). This behavior limits their ability to locate optimal sardine habitat (21), enhances predation of the sardine eggs and larvae (22), and potentially may halt their seasonal feeding migration, again with deleterious effects on their condition. We hypothesize that the low sardine biomass and their increased propensity to mix with other species creates a negative-feedback mechanism that serves to accelerate the decline of the population and greatly limits the possibilities for a near-term recovery.
Currently, the exploitation of sardine off the west coast of North America is at the highest possible rate within the management framework (25), and the largest, most fecund fish have been targeted increasingly despite clear indications of their depletion (Figs. 2C and 3). The harvest guidelines are based on a positive relationship between sea-surface temperature and sardine productivity observed in data from the previous warm cycle (25). That relationship, however, does not hold in the current state of the ecosystem (40), and our analysis of the stock recruitment indicates that sardine recruitment is currently density dependent and is affected positively by the condition of the parental population before the spawning season (Table S1). Succinctly, the current decline in the northern sardine stock in the CCE is the result of the high exploitation rates of a stock with limited productivity since 2003, coincident with a transition into a cold period.
In contrast to the fishery-independent biomass indices, which show precipitous declines, total sardine landings indicate only slight signs of stock biomass recession (Fig. 2A). Perhaps, irrespective of the fishery, the effects of changes in the environment are inescapable, and the sardine stock is fated to collapse again (15). Perhaps, as in the past, the residual sardine stock should be fished until continued fishing is not economically viable (6). Although economically attractive in the short term, this strategy may have long-term deleterious effects on the numerous natural predators of sardine and on the speed of the sardine-stock recovery during the next warm period (33). In addition to being prey for mackerel, sardine are prey for Pacific hake (Merluccius productus), multiple depleted species of shark, tuna, salmon, marlin, and barracuda, and many species of seabirds and marine mammals (41). Also, if the residual seed stock of sardine is too small, the fish may not form single-species aggregations and find optimal foraging and reproductive habitat, and their behavioral and phenotypic diversity may be insufficient for resilience during unstable environmental conditions (8). For example, the onset, size, and duration of the recent sardine fishery all were apparently affected adversely by persistent fishing of the declining stock in the mid-1900s (Fig. 2 A and C).
As an alternative to the above strategy, considering the current stock size, distribution, demographics, and condition estimated directly from acoustic-trawl surveys (10, 11) and the cyclical environmental periods witnessed by the PDO and other indicators, the exploitation rates could be set conservatively. Jacobson and MacCall (42) indicated that during persistent periods of adverse environmental conditions (e.g., cold seawater temperatures), little or no sardine harvest may be sustainable. Such a precautionary approach might allow a seed stock to persist until, and recruit quickly in, another warm period and thereby reduce uncertainty in social and business decisions in both the short and long term.
In the short term, the success of the northern sardine stock in the CCE appears to depend on the strength and management of the modest number of sardine recruits detected in the 2011 acoustic-trawl survey. In the medium term, sardine will have to struggle with more unstable and colder oceanographic conditions and the increasing predation and competition of resurging epipelagic species. In the long term, the condition and size of the northern sardine stock in the CCE may well depend on the management actions taken now.
The management strategies for Pacific sardine must be in accordance with the 1976 Magnuson Fishery Conservation and Management Act and subsequent reauthorizations and amendments (43). In particular, current management actions must consider “the rate or level of fishing mortality that jeopardizes the capacity of a fishery to produce the maximum sustainable yield on a continuing basis.” To do so, assessments rely on long-term averages of estimated fishing and natural mortality and recruitment success (44). However, such long-term stability is not exhibited naturally by populations of Pacific sardine (8, 42) and other small pelagic fishes (5, 45). Consequently, management strategies that maximize yield during periodic population increases may accelerate the periodic population declines. Therefore, different management objectives and procedures may be required to avoid overfishing of short-lived, environmentally dependent pelagic fish species. We advocate that stock assessment and management of sardine and other small pelagic fishes consider the short term sustainability of the stock based on direct measurements of mortality (natural and fishing) and recruitment strength evaluated through the results of frequent acoustic-trawl surveys (10, 11), together with an evaluation of the cyclical environmental conditions [e.g., as indicated by the PDO (14, 16)].
Conclusion
The exact cause of the collapse of the northern sardine stock in the CCE in the mid-1900s remains elusive (17). However, our analysis of the literature and the results of our 2006–2011 acoustic-trawl surveys (10, 11) indicate that many environmental and anthropogenic characteristics of the collapse appear to be recurring. All indicators show that the northern sardine stock off the west coast of North America is declining steeply again and that imminent collapse is likely.
Our acoustic-trawl surveys (10, 11) have provided unique foresight of this ecological and socioeconomic juncture. Based on the results of our spring 2011 survey of multiple pelagic fish species, the dominant species of small pelagic fishes in the CCE has changed, for the near future, to Pacific mackerel. In the past, the importance of such indicators was recognized only after striking repercussions in the fishery had occurred (6, 8). By then, the path of the northern Pacific sardine stock was irreversible, and the recovery was protracted (6).
Materials and Methods
Monthly values of the PDO (http://jisao.washington.edu/pdo/PDO.latest) (Fig. 2C) (18), starting in 1900, were averaged by year, and a sinusoidal oscillation with a 60-y period, identified by spectral analysis, was fitted to the time series by least-squares minimization. Then the trend of the PDO from 2011–2020 was forecast.
Indices of the sardine spawning stock biomass (Fig. 3) were obtained from the 2010 stock assessment (25), from the daily egg production method (DEPM) annual survey reports (ref. 39 and references therein), and from our acoustic-trawl surveys (10, 11). Also using data from our surveys, Fig. 4C was constructed by calculating the proportions of sardine and jack and Pacific mackerel in trawl catches containing one or more fish species, and the proportion of trawls in which sardine dominated (where sardine catch by weight was greater than 90% of all of the epipelagic fish catch) in the survey trawls from 2004–2011 (ref. 39 and references therein). Recruitment indices used in Fig. 4A and Table S1 are in ref. 25. Mean condition factors were calculated by averaging the individual condition factors (K = 105 *weight *length−3; where weight is in grams and length is in millimeters) (34) of sardine larger than 19 cm (standard length) obtained from the Oregon and Washington fisheries during the feeding season before the recruit's year-class (data courtesy of the Oregon and Washington Departments of Fish and Wildlife).
Sardine biomass densities were estimated from our surveys of the CCE conducted using the acoustic-trawl method (10, 11) in spring 2006, 2008, 2010, and 2011 (Fig. 5) and were analyzed in the context of our verified modeled of sardine habitat (Fig. 1) and migration (9, 10). Acoustic densities from echoes of schooling epipelagic species were apportioned to the species present in the trawls, considering their abundances and length compositions. Sardine length distributions, weighted by their relative abundance in the survey area, were derived from the trawl catches.
Supplementary Material
Acknowledgments
We thank the staff from the National Marine Fisheries Service, the Scripps Institution of Oceanography, and State Agencies that collected, processed, and archived data from the daily egg production method, California Cooperative Oceanic Fisheries Investigations, and market sampling surveys. We thank Kevin Hill of the Southwest Fisheries Science Center (SWFSC) for providing the assessment-derived estimates of sardine abundance and recruitment. We thank Russ Davis of the Scripps Institution of Oceanography for his very helpful suggestions for improving the organization and style of this paper, particularly regarding the use of first person to highlight the critical contributions of our acoustic-trawl survey results to this investigation. We thank three anonymous reviewers for their constructive comments. J.P.Z.'s postdoctoral internship with D.A.D. at the SWFSC was partially funded by the Portuguese Foundation for Science and Technology (SFRH/BPD/44834/2008) and by the Fisheries Resource Division (Russ Vetter, Director).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113806109/-/DCSupplemental.
References
- 1.Worm B, et al. Rebuilding global fisheries. Science. 2009;325:578–585. doi: 10.1126/science.1173146. [DOI] [PubMed] [Google Scholar]
- 2.Finney BP, Gregory-Eaves I, Douglas MSV, Smol JP. Fisheries productivity in the northeastern Pacific Ocean over the past 2,200 years. Nature. 2002;416:729–733. doi: 10.1038/416729a. [DOI] [PubMed] [Google Scholar]
- 3.Anderson CNK, et al. Why fishing magnifies fluctuations in fish abundance. Nature. 2008;452:835–839. doi: 10.1038/nature06851. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner T, Soutar A, Ferreira-Bartrina V. Reconstruction of the history of Pacific sardine and northern anchovy populations over the past two millennia from sediments of the santa Barbara Basin, California. Cal Coop Ocean Fish. 1992;33:24–40. [Google Scholar]
- 5.Pinsky ML, Jensen OP, Ricard D, Palumbi SR. Unexpected patterns of fisheries collapse in the world's oceans. Proc Natl Acad Sci USA. 2011;108:8317–8322. doi: 10.1073/pnas.1015313108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radovich J. The collapse of the California sardine fishery: What have we learned? Cal Coop Ocean Fish. 1982;23:56–78. [Google Scholar]
- 7.Félix-Uraga R, et al. Pacific sardine (Sardinops sagax) stock discrimination off the west coast of Baja California and southern California using otolith morphometry. Cal Coop Ocean Fish. 2005;46:113–121. [Google Scholar]
- 8.Murphy GI. Vital statistics of Pacific sardine (Sardinops Caerulea) and population consequences. Ecology. 1967;48:731–736. doi: 10.2307/1933730. [DOI] [PubMed] [Google Scholar]
- 9.Zwolinski JP, Emmett RL, Demer DA. Predicting habitat to optimize sampling of Pacific sardine (Sardinops sagax) ICES J Mar Sci. 2011;68:867–879. [Google Scholar]
- 10.Demer DA, et al. Seasonal migration of Pacific sardine (Sardinops sagax) in the California Current ecosystem: Prediction and empirical confirmation. Fish B-Noaa. 2012;110:52–70. [Google Scholar]
- 11.Zwolinski JP, et al. Distributions and abundances of Pacific sardine (Sardinops sagax) and other pelagic fishes in the California Current ecosystem during spring 2006, 2008, and 2010, estimated from acoustic-trawl surveys. Fish B-Noaa. 2012;110:110–122. [Google Scholar]
- 12.Schwartzlose RA, et al. Worldwide large-scale fluctuations of sardine and anchovy populations. S Afr J Mar Sci. 1999;21:289–347. [Google Scholar]
- 13.Clark FN, Janssen JF., Jr Movements and abundance of the sardine as measured by tag returns. California Division of Fish and Game. Fish Bull. 1945;61:7–42. [Google Scholar]
- 14.Alheit J, Bakun A. Population synchronies within and between ocean basins: Apparent teleconnections and implications as to physical-biological linkage mechanisms. J Mar Syst. 2010;79:267–285. [Google Scholar]
- 15.MacCall AD. Patterns of low-frequency variability in fish populations of the California Current. Cal Coop Ocean Fish. 1996;37:100–110. [Google Scholar]
- 16.Chavez FP, Ryan J, Lluch-Cota SE, Niquen C M. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science. 2003;299:217–221. doi: 10.1126/science.1075880. [DOI] [PubMed] [Google Scholar]
- 17.Maccall AD. Mechanisms of low-frequency fluctuations in sardine and anchovy populations. In: Checkley DM Jr, Alheit J, Oozeki Y, Roy C, editors. Climate Change and Small Pelagic Fish. New York: Cambridge Univ Press; 2009. pp. 285–299. [Google Scholar]
- 18.Mantua N, Hare SR, Zhang Y, Wallace JM, Francis RC. A Pacific decadal climate oscillation with impacts on salmon. Bull Am Meteorol Soc. 1997;78:1069–1079. [Google Scholar]
- 19.Barnes JT, MacCall AD, Jacobson LD, Wolf P. Recent population trends and abundance estimates for the Pacific sardine (Sardinops Sagax) Cal Coop Ocean Fish. 1992;33:60–75. [Google Scholar]
- 20.MacCall AD. Population estimates for the warming years of the Pacific sardine fishery. Cal Coop Ocean Fish. 1979;20:72–82. [Google Scholar]
- 21.Bakun A, Cury P. The “school trap”: A mechanism promoting large-amplitude out-of-phase population oscillations of small pelagic fish species. Ecol Lett. 1999;2:349–351. [Google Scholar]
- 22.Mearns AJ, Young DR, Olson RJ, Schafer HA. Trophic structure and the cesium-potassium ratio in pelagic ecosystems. Cal Coop Ocean Fish. 1981;22:99–110. [Google Scholar]
- 23.Nøttestad L, Giske J, Holst JC, Huse G. A length-based hypothesis for feeding migrations in pelagic fish. Can J Fish Aquat Sci. 1999;56:26–34. [Google Scholar]
- 24.Smith PE, Moser HG. Long-term trends and variability in the larvae of Pacific sardine and associated fish species of the California Current region. Deep Sea Res Part II Top Stud Oceanogr. 2003;50:2519–2536. [Google Scholar]
- 25.Hill KT, Lo NCH, Macewicz BJ, Crone PR, Felix-Uraga R. 2010. Assessment of the Pacific Sardine Resource in 2010 for U.S. Management in 2011. National Oceanic and Atmospheric Administration Technical Memorandum NMFS-SWFSC-469 (US Department of Commerce, Washington, DC). Available at http://swfsc.noaa.gov/publications/TM/SWFSC/NOAA-TM-NMFS-SWFSC-469.pdf. Accessed February 9, 2012.
- 26.Beamish RJ, et al. The regime concept and natural trends in the production of Pacific salmon. Can J Fish Aquat Sci. 1999;56:516–526. [Google Scholar]
- 27.Irvine JR, Fukuwaka MA. Pacific salmon abundance trends and climate change. ICES J Mar Sci. 2011;68:1122–1130. [Google Scholar]
- 28.Fisheries and Oceans Canada (DFO) Evaluation of Pacific sardine (Sardinops sagax) stock assessment and harvest guidelines in British Columbia. Science Advisory Report 2011/016. 2011. Available at http://www.dfo-mpo.gc.ca/csas-sccs/Publications/SAR-AS/2011/2011_016-eng.pdf. Accessed February 9, 2012.
- 29.Schweigert JF, MacFarlane G, Hodes V. Pacific Sardine (Sardinops Sagax) Biomass and Migration Rates in British Columbia. Fisheries and Oceans Canada (DFO) Sci. Advis. Res. Doc. 2009/088. 2009. Available at http://www.dfo-mpo.gc.ca/CSAS/Csas/publications/resdocs-docrech/2009/2009_088_e.pdf. Accessed February 9, 2012.
- 30.Francis RC, Hare SR. Decadal-scale regime shifts in the large marine ecosystem of the north-east Pacific: A case for historical science. Fish Oceanogr. 1994;3:279–291. [Google Scholar]
- 31.Rykaczewski RR, Checkley DM., Jr Influence of ocean winds on the pelagic ecosystem in upwelling regions. Proc Natl Acad Sci USA. 2008;105:1965–1970. doi: 10.1073/pnas.0711777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McFarlane GA, Beamish RJ. The re-occurrence of sardines off British Columbia characterizes the dynamic nature of regimes. Prog Oceanogr. 2001;49:151–165. [Google Scholar]
- 33.Petitgas P, Secor DH, McQuinn I, Huse G, Lo N. Stock collapses and their recovery: Mechanisms that establish and maintain life-cycle closure in space and time. ICES J Mar Sci. 2010;67:1841–1848. [Google Scholar]
- 34.Ricker WE. Computation and Interpretation of Biological Statistics of Fish Populations. Ottawa: Fisheries and Marine Service; 1975. [Google Scholar]
- 35.Beverton RJH. Small marine pelagic fish and the threat of fishing - are they endangered. J Fish Biol. 1990;37:5–16. [Google Scholar]
- 36.Mullon C, Fréon P, Cury P. The dynamics of collapse in world fisheries. Fish Fish. 2005;6:111–120. [Google Scholar]
- 37.Frank KT, Brickman D. Allee effects and compensatory population dynamics within a stock complex. Can J Fish Aquat Sci. 2000;57:513–517. [Google Scholar]
- 38.Shertzer KW, Prager MH. Delay in fishery management: Diminished yield, longer rebuilding, and increased probability of stock collapse. ICES J Mar Sci. 2007;64:149–159. [Google Scholar]
- 39.Lo N, Macewicz BJ, Griffith DA. Washington, DC: US Department of Commerce; 2010. Spawning Biomass of Pacific Sardine (Sardinops sagax) of U.S. in 2010. National Oceanic and Atmospheric Administration Technical Memorandum NMFS-SWFSC-463. Available at http://swfsc.noaa.gov/publications/TM/SWFSC/NOAA-TM-NMFS-SWFSC-463.pdf. Accessed February 9, 2012. [Google Scholar]
- 40.McClatchie S, Goericke R, Auad G, Hill K. Re-assessment of the stock-recruit and temperature-recruit relationships for Pacific sardine (Sardinops sagax) Can J Fish Aquat Sci. 2010;67:1782–1790. [Google Scholar]
- 41.Emmett RL, et al. Pacific sardine (Sardinops sagax) abundance, distribution, and ecological relationships in the Pacific Northwest. Cal Coop Ocean Fish. 2005;46:122–143. [Google Scholar]
- 42.Jacobson LD, MacCall AD. Stock recruitment models for Pacific sardine (Sardinops-Sagax) Can J Fish Aquat Sci. 1995;52:2062–2062. [Google Scholar]
- 43.Tromble GR, Lambert DM, Lee LR. 2009. Prelude to sustainability: Ending overfishing in U.S. fisheries. Our Living Oceans. Report on the Status of U.S. Living Marine Resources. National Oceanic and Atmospheric Administration Technical Memorandum NMFS-F/SPO-80) (US Department of Commerce), 6th Ed, pp 57–66. Available at http://spo.nwr.noaa.gov/olo6thedition/01--Cover%20through%20Title%20Page.pdf. Accessed February 9, 2012.
- 44.Hilborn R, Walters CJ. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty. New York: Chapman and Hall; 1992. [Google Scholar]
- 45.Watanabe Y, Zenitani H, Kimura R. Population decline of the Japanese sardine Sardinops melanostictus owing to recruitment failures. Can J Fish Aquat Sci. 1995;52:1609–1616. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.