Abstract
Abstact
Background
Gamma amino butyric acid (GABA), the principal inhibitory neurotransmitter in the cerebral cortex, maintains the inhibitory tones that counter balances neuronal excitation. When this balance is perturbed, seizures may ensue.
Methods
In the present study, alterations of the general GABA, GABAA and GABAB receptors in the cerebral cortex of the epileptic rat and the therapeutic application of Bacopa monnieri were investigated.
Results
Scatchard analysis of [3H]GABA, [3H]bicuculline and [3H]baclofen in the cerebral cortex of the epileptic rat showed significant decrease in Bmax (P < 0.001) compared to control. Real Time PCR amplification of GABA receptor subunits such as GABAAά1, GABAAγ, GABAAδ, GABAB and GAD where down regulated (P < 0.001) in epileptic rats. GABAAά5 subunit and Cyclic AMP responsible element binding protein were up regulated. Confocal imaging study confirmed the decreased GABA receptors in epileptic rats. Epileptic rats have deficit in radial arm and Y maze performance.
Conclusions
Bacopa monnieri and Bacoside-A treatment reverses epilepsy associated changes to near control suggesting that decreased GABA receptors in the cerebral cortex have an important role in epileptic occurrence; Bacopa monnieri and Bacoside-A have therapeutic application in epilepsy management.
Keywords: Epilepsy, Bacopa monnieri, Bacoside-A, Pilocarpine, Carbamazepine
Background
GABA is formed within GABAergic axon terminals and released into the synapse, where it acts at one of two types of receptor GABAA which controls chloride entry into the cell, and GABAB, which increases potassium conductance, decreases calcium entry, and inhibits the presynaptic release of other neurotransmitters [1,2]. Temporal lobe epilepsy (TLE) is considered the most common epileptic syndrome and it is estimated that approximately 80% of patients with partial seizures have temporal lobe epilepsy [3]. The effect of pilocarpine treatment, which is characterized by generalized convulsive status epilepticus in rodents, well represents the characteristic neuropathological findings in the brain regions of the patients with TLE epilepsy [4]. Imbalance between excitatory and inhibitory synaptic transmission in key brain areas are implicated in the pathophysiology of TLE, in which there is a decrease in the GABA mediated inhibition. TLE seizures reflect excess excitation, which result from local inhibitory circuit dysfunction. The existence of multiple GABAA receptor subtypes differing in subunit composition, localization and functional properties underlies their role for fine-tuning of neuronal circuits and genesis of network oscillations. The differential regulation of GABAA receptor subtypes represents a major facet of homeostatic synaptic plasticity and contributes to the excitation/inhibition (E/I) balance under physiological conditions and upon pathological challenges [5]. Glutamate decarboxylase (GAD) is an enzyme that catalyzes the decarboxylation of glutamate to GABA. GAD is the rate limiting enzyme of GABA synthesis and it is used as a marker for GABAergic activity [6]. cAMP response element-binding protein (CREB) is a transcription factor that has been implicated in the activation of protein synthesis required for long-term memory and seizure formation [7]. Findings shows that GABAB receptor subunit gene expression in hippocampal neurons is mediated through the CREB by binding to unique cAMP response elements in the alternative promoter regions [8]. Radial arm maze is a means to examine the neural systems that are involved in memory and the influence of pharmacological compounds on memory [9,10]. Y-maze used to evaluate the spatial learning by the different animal models of the rats [11]
The potential for antiepileptic drugs to negatively impact cognitive abilities is of significant concern because they are the major therapeutic modality for control of seizures. An increased risk for cognitive deficits has been noted in patients with temporal lobe seizures. Moreover, many of the anticonvulsant drugs presently used for treating epilepsy cannot prevent neurodegeneration but rather contribute towards these cognitive deficits [12,13]. The available anti-epileptic drugs are not curative since they mostly treat the symptoms of the disease and render little help to alleviate its cause. This has instilled a renewed interest in traditionally used herbal drugs and formulations, which are safe in prolonged usage for the management of epilepsy.
Bacopa monnieri is well known for its neuropharmacological effects. It is currently recognized as being effective in the treatment of mental illness and epilepsy [14]. Treatments with Bacopa monnieri extract [15] have enhanced learning ability. Cognition-facilitating effect was due to two active saponins, bacosides A and B present in the ethanol extract [16]. These active principles, apart from facilitating learning and memory in normal rats, inhibited the amnesic effects of scopolamine, electroshock and immobilization stress. Crude plant extract or bacosides have also shown anxiolytic effects, antidepressant activity, anticonvulsive action and antioxidant activity [17]. In vitro studies using Bacopa monnieri have been found to inhibit free radical formation and DNA damage in a dose dependent manner [14]. But so far there are very few studies reporting the role of Bacopa monnieri treatment on the functional regulation of GABA receptors. In this study, we investigated the anti-epileptic effect of Bacopa monnieri on the GABAergic receptor binding, gene expression in the cerebral cortex and spatial learning and memory in epileptic rats.
Methods
Chemicals Used in the Study
Biochemicals used in the present study were purchased from Sigma Chemical Co., St. Louis, USA. All other reagents were of analytical grade purchased locally [3H]GABA [3H]bicuculline and [3H]baclofen were purchased from NEN life sciences products Inc., Boston, U.S.A.
Animals
Adult Wistar rats of 250-300 g body weight purchased from Amrita Institue of Medical Sciences, Cochin and Kerala Agriculture University, were used for all experiments. They were housed in separate cages under 12 hrs light and 12 hrs dark periods and were maintained on standard food pellets and water adlibitum. All animal care and procedures were taken in accordance with the Institutional, National Institute of Health and CPESCA guidelines.
Plant material and Preparation of extract
Specimen of Bacopa monnieri (L.) Pennel were collected from Cochin University area and were taxonomically identified and authenticated by Mr. K. P. Joseph, Head, Dept. of Botany (retd.), St. Peter's College, Kollenchery and voucher specimens was deposited at a herbarium (No: MNCB3) of Centre for Neuroscience, Cochin University of Science and Technology, Cochin, Kerala, India. Fresh, whole Bacopa monnieri plant were collected and washed. Leaves, roots and stems of Bacopa monnieri plant were cut into small pieces and dried in shade. 100 g fresh plant dried in shade yielded approximately 15 g powder. Homogenate was extracted at required concentration (300 mg fresh plant/kg body weight) by dissolving 450 mg of dried powder in 80 ml distilled water and used for the study [18,19].
Induction of Epilepsy in adult rats
Adult male Wistar rats, weighing 250 to 300 g, were housed for 1 to 2 weeks before experiments were performed. Epilepsy was induced by injecting rats with pilocarpine (350 mg/kg body weight i.p.), preceded by 30 min with atropine (1 mg/kg body weight i.p.) to reduce peripheral pilocarpine effects. Within 20 to 40 min after the pilocarpine injection, essentially all the animals developed status epilepticus (SE). Control animals were given saline injection. Behavioural observation continued for 5 hrs after pilocarpine injection. SE was allowed to continue for 1 hr and then control and experimental animals were treated with diazepam (4 mg/kg body weight i.p.). Animals recovered from this initial treatment within 2 to 3 days and were observed for the next 3 weeks. 24 days after pilocarpine treatment, the rats were continuously video monitored for 72 hrs. The behaviour and seizures were captured with a CCD camera and a Pinnacle PCTV capturing software card. One trained technician, blind to all experimental conditions, viewed all videos. Seizure activity was rated according to Racine Scale [20]. Seizures were assessed by viewing behavioural postures (i.e. lordosis, straight tail, jumping/running, forelimb clonus and/or rearing) during observation of the videos. Experimental rats which showed recurrent seizures were used for the further experiments. Experimental rats were divided into five groups: 1) Control (C), 2) Epileptic (E), 3) Epileptic rats treated with Bacopa monnieri (E+B), 4) Epileptic rats treated with bacoside-A (E+D), 5) Epileptic rats treated with carbamazepine (E+C). Bacopa monnieri treated rats were given extract of Bacopa monnieri orally in the dosage 300 mg/kg body weight/day for 15 days. Carbamazepine- a standard drug used for the treatment of epilepsy was given orally in the dosage 150 mg/kg body weight/day for 15 days. Bacoside-A was given orally in the dosage 150 mg/kg body weight/day for 15 days. After the treatment the rats were sacrificed and the tissues were stored in -80°C.
Protein Determination
Protein was measured by the method of Lowry et al [21] using bovine serum albumin as standard. The intensity of the purple blue colour formed was proportional to the amount of protein, which was read in a spectrophotometer at 660 nm.
GABA Receptor Binding Assay
[3H]GABA binding to GABA, [3H]bicuculline to GABAA and [3H]baclofen binding to GABAB receptors were assayed in triton x-100 treated synaptic membranes according to the procedure of Kurioka et al [22]. Crude synaptic membrane was prepared using sodium-free 10 mM Tris buffer (PH7.4). Each assay tube contained a protein concentration of 100 mg. In saturation binding experiments, 5-40 nM of [3H] GABA incubated with and without excess of unlabelled GABA (100 μM). The incubation was continued for 20 min at 40°C and terminated by centrifugation at 35000 xg for 20 min. [3H]GABA in the pellet was determined by liquid scintillation spectrometry. Specific binding was determined by subtracting non-specific binding from the total binding.
Real-Time Polymerase Chain Reaction
Total RNA was isolated from the cerebral cortex of control and experimental rats using Tri reagent. RNA was reverse transcribed using ABI PRISM cDNA Archive kit. 20 μl of the reaction mixture contained 0.2 μg total RNA, 10X RT buffer, 25X dNTP mixture, 10X Random primers, MultiScribe RT (50 U/μl) and RNAase free water. The reactions were carried out at 25°C for 10 minutes and 37°C for 2 hours using an Eppendorf Personal Cycler. Real-Time PCR assays were performed using specific primer and fluorescently labeled Taq probe in an ABI 7300 Real-Time PCR instrument (Applied Biosystems). The TaqMan reaction mixture of 20 μl contained 25 ng of total RNA-derived cDNAs, 200 nM each of the forward primer, reverse primer and TaqMan probe for specific gene, endogenous control, β-actin and 12.5 μl of TaqMan 2X Universal PCR Mastermix. The thermocycling profile conditions used were: 50°C - 2 minutes - Activation; 95°C - 10 minutes - Initial Denaturation; followed by 40 cycles of 95°C - 15 seconds - Denaturation and 60°C-1 minute - Annealing. The ΔΔCT method of relative quantification was used to determine the fold change in expression. This was done by first normalizing the resulting threshold cycle (CT) values of the target mRNAs to the CT values of the internal control β-actin in the same samples (ΔCT = CTTarget - CTβ-actin). It was further normalized with the control (ΔΔCT = ΔCT - CT Control). The fold change in expression was then obtained as (2-ΔΔCT) and expressed as log 2-ΔΔCT.
The sequences of the probe were
GABAAά1 :- ATTTGGGAGCTATGCTTATACAAGA
GABAAά5 :- CAGCACCAGCACAGGTGAATATACA
GABAAγ3 :- GCATGCTCGGTCCAGGAGGGTGGAA
GABAAδ :- CCGCACCATGGCGCCAGAGCAATGA
GAD65:- AGTCATTACAAATCT TGCCC
The sequences of the primers were
GABAAά1 5' ACA AGA AGC CAG AGA ACA AGC CAG 3'
5' GAG GTC TAC TGG TAA GCT CTA CCA 3'
GABAAά5 5' TGA GAT GGC CAC ATC AGA AGC AGT 3'
5' TCA TGG GAG GCT GGA GTT TAG TTC 3'
GABAAγ3 5' CAG AGA CAG GAA GCT GAA AAG CAA 3'
5' CGA AGT GAT TAT ATT GGA CTA AGC 3'
GABAAδ 5' TGT GAG CAA CCG GAA ACC AAG CAA-3'
5' CGT GTG ATT CAG CGA ATA AGA CCC-3'
GAD65 5'GCCCAGGCTCATCGCATTCA-3'
5'AGTCATTACAAATCT TGCCC-3'
Linear regression analysis for Scatchard plots
The data were analysed according to Scatchard [23]. The specific binding was determined by subtracting non-specific binding from the total. The binding parameters, maximal binding (Bmax) and equilibrium dissociation constant (Kd), were derived by linear regression analysis by plotting the specific binding of the radioligand on X-axis and bound/free on Y-axis. The maximal binding is a measure of the total number of receptors present in the tissue and the equilibrium dissociation constant is the measure of the affinity of the receptors for the radioligand. Kd is inversely related to receptor affinity.
Radial Maze Test
Radial maze behavioral testing was conducted under normal room lighting and utilized an eight armed radial maze elevated 100 cm from the floor. Each arm of the maze (11.5 cm wide) extended 68.5 cm from an octagonally shaped central platform (40 cm across). Black Plexiglas walls (11.5 cm high) were present only for the first 20 cm of each arm to prevent the rat crossing from one to another without returning to the central platform. Circular food wells (1.3 cm deep, 3.2 cm diameter) were located 2.5 cm from the end of each arm. The maze was centered in an enclosed room where lighting and spatial cues (e.g., posters, door, and boxes) remained constant throughout the course of the experiment. Arms were baited by placing one raisin in each food well.
Rats were placed on the maze 3 days prior to the start of formal acquisition testing in order to habituate them to the apparatus. On the first day of habituation, 4 food pellets were scattered along the length of each arm. The rats were then systematically confined to each arm for 1 min to ensure their exposure to the entire maze. On the second day of habituation, the previous day's procedure was repeated except that the animals were not confined to each arm following 5 min of exploration. On the third day, one food pellet was placed in the food well at the end of each arm and a second was placed halfway down each arm. Once the rats were habituated to the maze, testing began. Trials began by placing a single rat in the center of the maze facing away from the experimenter. The trial ended when the rat had obtained all 4 pellets or 5 min had elapsed, whichever occurred first. Rats were run until they achieved criterion performance for task acquisition. Criterion was attained when the rat collected 3 out of the 4 food pellets within their first 4 arm entries within a trial (while still completing the trial) with this level of performance being maintained for 5 consecutive criterion performance. The number of trials up to and including the last of these 5 criterion performance formed the "number of trials to criterion" measure. Experimental subjects were tested under blind conditions. The time of testing was consistent from day to day for each subject but testing of the various treatment groups was distributed randomly throughout the day.
Y-Maze Test
The Y-maze was made of grey wood, covered with black paper, and consisted of three arms with an angle of 120°between each of the arms. Each arm was 8 cm width ×30 cm length ×15 cm height. The three identical arms were randomly designated: Start arm, in which the mouse started to explore (always open); Novel arm, which was blocked at the 1st trial, but open at the 2nd trial; and the other arm (always open). The maze was placed in a separate room with enough light. The floor of the maze was covered with sawdust, which was mixed after each individual trial in order to eliminate olfactory stimuli. Visual cues were placed on the walls of the maze.
The Y-maze test consisted of two trials separated by an inter-trial interval (ITI). The first trial (training) was 10 min duration and allowed the mouse to explore only two arms (start arm and the other arm) of the maze, with the third arm (novel arm) blocked. After a 1 h ITI [24], the second trial (retention) was conducted, during which all three arms were accessible and novelty vs. familiarity was analyzed through comparing behavior in all three arms. For the second trial, the mouse was placed back in the maze in the same starting arm, with free access to all three arms for 5 min. The time spent in each arm were analyzed data were expressed as percentage of performance in all three arms during the 5-minutes of test [25].
Confocal imaging
Control and experimental rats were deeply anesthetized with ether. The rat was transcardially perfused with PBS (pH- 7.4) followed by 4% paraformaldehyde in PBS [26]. After perfusion the brains were dissected and immersion fixed in 4% paraformaldehyde for 1 hr and then equilibrated with 30% sucrose solution in 0.1 M PBS. 40 μm sections were cut using Cryostat (Leica, CM1510 S). The sections were treated with PBST (PBS in 0.05% Triton X-100) for 20 min. Brain slices were incubated overnight at 4°C with rat primary antibody for GABAAα1. The brain slices were then rinsed with PBST and then incubated with Rhodamine coated secondary antibody. The sections were observed and photographed using confocal imaging system (Leica SP 5).
Results
Scatchard analysis of [3H]GABA binding against GABA in the cerebral cortex of Control, Epileptic, Epileptic + Bacopa monnieri, Epileptic + Bacoside A, and Epileptic + Carbamazepine treated rats
Scatchard analysis of [3H]GABA against GABA in the cerebral cortex showed a significant decrease (P < 0.001) in the Bmax of epileptic rats compared to controls. Kd showed significant decrease (P < 0.05) in the epileptic group compared to control. Treatment using Bacopa monnieri, Bacocide-A and Carbamazepine reversed the Bmax to near control (Table 1).
Table 1.
Scatchard analysis of [3H]GABA binding against GABA in the cerebral cortex of control and experimental rats
| Group | Bmax (fmoles/mg ptn) | Kd (nM) |
|---|---|---|
| C | 112.3 ± 6.3 | 2.6 ± 0.3 |
| E | 44.0 ± 3.5*** | 1.5 ± 0.1** |
| E+B | 89.8 ± 5.6@@ | 2.3 ± 0.2 |
| E+D | 93.2 ± 4.7@@ | 2.2 ± 02 |
| E+C | 80.7 ± 5.2@@ | 2.1 ± 0.1 |
Values are mean ± SEM of 4-6 separate experiments. Each group consist of 6-8 rats ** p < 0.01, *** p < 0.001 when compared to control, @@ < 0.01, @@@ < 0.001 when compared to epileptic group. C- Control, E- Epileptic, E+B- Epileptic rats treated with Bacopa monnieri, E+D- Epileptic rats treated with Bacoside A and E+C- Epileptic rats treated with Carbamazepine.
Scatchard analysis of [3H]bicuculline against Bicuculline and [3H]baclofen against baclofen in the cerebral cortex of Control, Epileptic, Epileptic + Bacopa monnieri, Epileptic + Bacoside A and Epileptic + Carbamazepine, treated rats
Scatchard analysis of [3H]bicuculline against bicuculline and [3H]baclofen against baclofen in the cerebral cortex showed a significant decrease (P < 0.001) in the Bmax of epileptic rats compared to controls. Kd showed significant decrease (P < 0.05) in epileptic group compared to control. Treatment using Bacopa monnieri, Bacocide-A and Carbamazepine reversed the Bmax to near control (Tables 2 and 3).
Table 2.
Scatchard analysis of [3H]bicuculline binding against bicuculline in the cerebral cortex of control and experimental rats
| Group | Bmax (fmoles/mg ptn) | Kd (nM) |
|---|---|---|
| C | 85.4 ± 4.5 | 1.7 ± 0.3 |
| E | 37.9 ± 3.9*** | 0.9 ± 0.1** |
| E+B | 75.0 ± 5.3@@@ | 1.2 ± 0.3 |
| E+D | 80.1 ± 6.1@@@ | 1.39 ± 0.1 |
| E+C | 76.5 ± 4.7@@@ | 1.38 ± 0.2 |
Values are mean ± SEM of 4-6 separate experiments. Each group consist of 6-8 rats ** p < 0.01, *** p < 0.001 when compared to control, @@ < 0.01, @@@ < 0.001 when compared to epileptic group. C- Control, E- Epileptic, E+B- Epileptic rats treated with Bacopa monnieri, E+D- Epileptic rats treated with Bacoside A and E+C- Epileptic rats treated with Carbamazepine.
Table 3.
Scatchard analysis of [3H]baclofen binding against Baclofen in the cerebral cortex of control and experimental rats
| Group | Bmax (fmoles/mg ptn) | Kd (nM) |
|---|---|---|
| C | 124.3 ± 8.5 | 1.8 ± 0.2 |
| E | 91.9 ± 6.9*** | 1.2 ± 0.1** |
| E+B | 115.2 ± 7.3@@@ | 1.6 ± 0.3 |
| E+D | 111.7 ± 5.1@@@ | 1.3 ± 0.1 |
| E+C | 107.1 ± 7.7@@@ | 1.4 ± 0.2 |
Values are mean ± SEM of 4-6 separate experiments. Each group consist of 6-8 rats ** p < 0.01, *** p < 0.001 when compared to control, @@ < 0.01, @@@ < 0.001 when compared to epileptic group. C- Control, E- Epileptic, E+B- Epileptic rats treated with Bacopa monnieri, E+D- Epileptic rats treated with Bacoside A and E+C- Epileptic rats treated with Carbamazepine.
Real Time-PCR analysis of GABAAά1, GABAAά5 GABAAγ and GABAAδ, receptor subunit mRNA in the cerebral cortex of Control, Epileptic, Epileptic + Bacopa monnier , Epileptic + Bacoside A and Epileptic + Carbamazepine treated rats
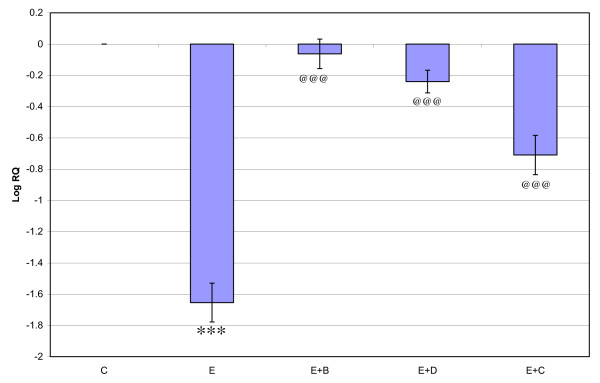
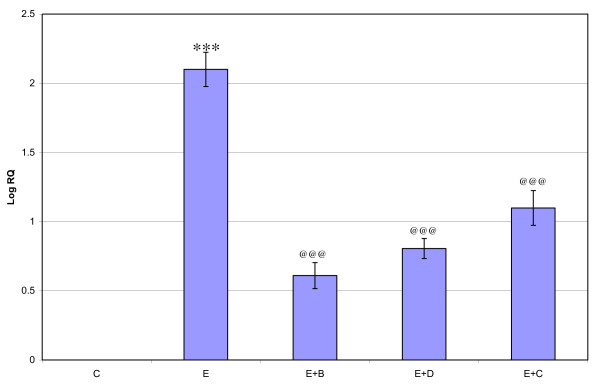
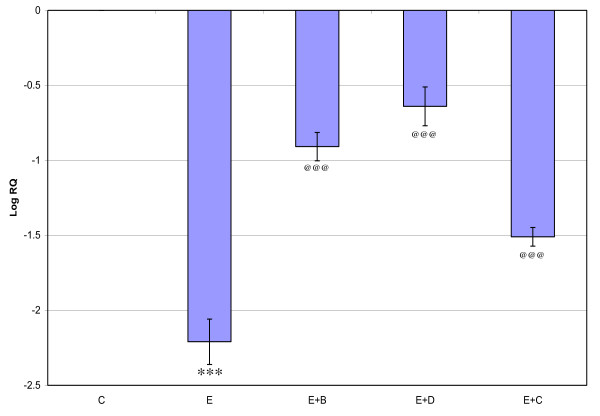
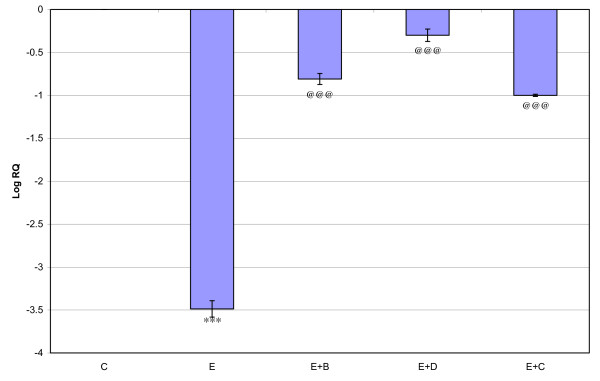
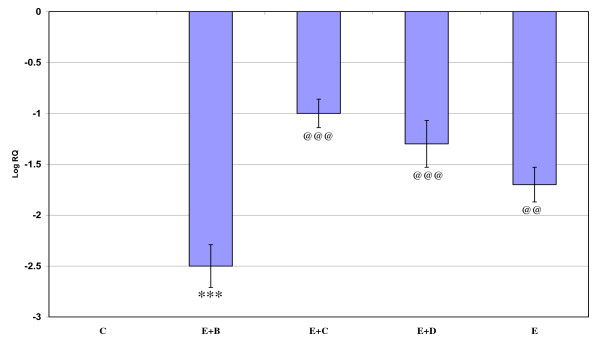
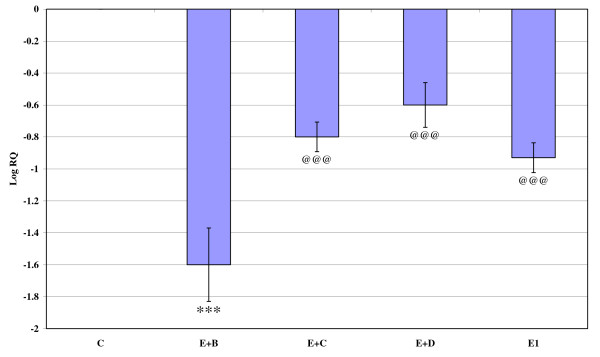
Gene expression of GABAAά1, GABAAγ and GABAAδ, where showed significant down regulation (p < 0.001) in the cerebral cortex of the epileptic rats compared to the control. Gene expression of GABAAά5 receptor subunit showed significant up regulation compared to the control. Treatment using Bacopa monnieri, Bacocide-A and Carbamazepine reversed the changes to near control (Figures 1, 2, 3 and 4).
Figure 1.
Representative graph showing Real-Time amplification of GABA Aα1 receptor subunit mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@@p < 0.001 when compared to epileptic group.
Figure 2.
Representative graph showing Real-Time amplification of GABAAα5 receptor subunit mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@@p < 0.001 when compared to epileptic group.
Figure 3.
Representative graph showing Real-Time amplification of GABA Aγ5 receptor subunit mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@@p < 0.001 when compared to epileptic group.
Figure 4.
Representative graph showing Real-Time amplification of GABA Aδ5 receptor subunit mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@@p < 0.001 when compared to epileptic group.
Real Time-PCR analysis of GABAB, GAD65 and CREB mRNA in the cerebral cortex of Control, Epileptic, Epileptic + Bacopa monnieri, Epileptic + Bacoside A and Epileptic + Carbamazepine treated Epileptic rats
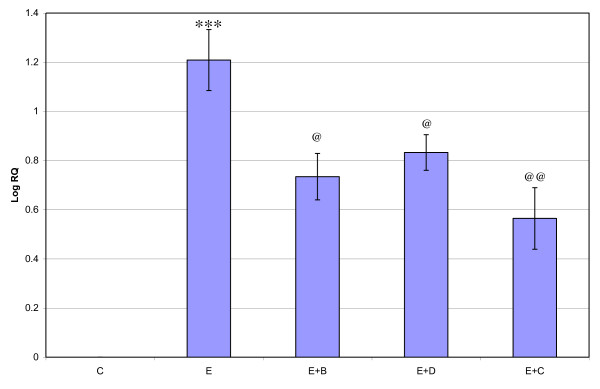
Gene expression of CREB mRNA showed significant up regulation (p < 0.001) in the cerebral cortex of the epileptic rats compared to the control. GABAB, and GAD65 mRNA were significantly down regulated compared to the control. Treatment using Bacopa monnieri, Bacoside-A and Carbamazepine were reversed the changes to near control (Figures 5, 6 and 7).
Figure 5.
Representative graph showing Real-Time amplification of GABAB receptor mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@@p < 0.001 when compared to epileptic group.
Figure 6.
Representative graph showing Real-Time amplification of GAD mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic+ Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@@p < 0.001 when compared to epileptic group.
Figure 7.
Representative graph showing Real-Time amplification of CREB mRNA from the Control and experimental rats. The ΔΔCT method of relative quantification was used to determine the fold change in expression. Values are mean ± S.D of 4-6 separate experiments. C- Control, E- Epileptic, E+B- Epileptic+ Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. ***p < 0.001 when compared to control, @@p < 0.01, @p < 0.05 when compared to epileptic group.
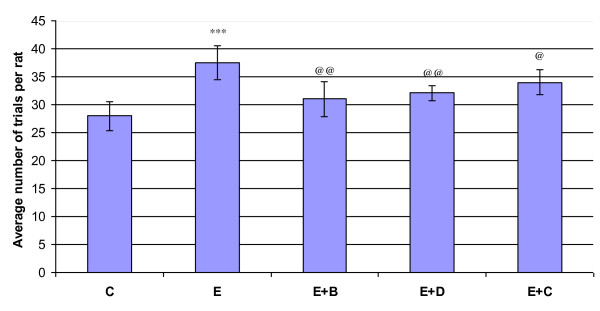
Eight-Arm Radial Maze Performance
There was significant increase (p < 0.001) in the number of trials required to achieve five consecutive criterion performances in the epileptic rats compared to control. Treatment using Bacopa monnieri and Bacoside-A reversed this change to near control (Figure 8).
Figure 8.
Representative graph showing radial arm maze performance of control and experimental rats epileptic rats required more daily trials to achieve three, four, and five consecutive criterion performances. Criterion performance was defined as consumption of the bait in the four baited arms of the radial maze during no more than five entries. There was no significant difference in the average number of trials required by kindled and control rats to achieve the initial criterion performance. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats.
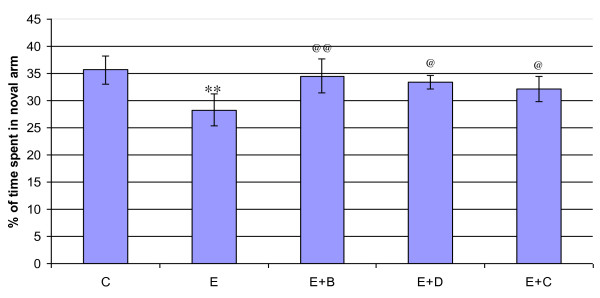
Y-Maze Performance
Time spent in the novel arm was decreed significantly (p < 0.001) in the epileptic group compared to control. Bacopa monnieri and Bacocide-A treated epileptic rats were showed improved performance (Figure 9).
Figure 9.
Representative graph showing Y maze performance of control and experimental rats. Epileptic rats showed less exploratory behavior compared to control. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. **p < 0.01 when compared to control, @@p < 0.01 and @p < 0.05 when compared to epileptic group.
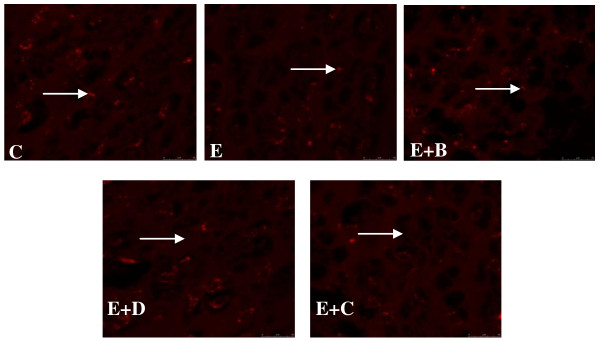
GABAAα1 receptor antibody staining in the Hippocampus of Control, Epileptic, Epileptic + Bacopa monnieri, Epileptic + Bacoside A and Epileptic + Carbamazepine treated Epileptic rats
GABAAα1 receptor subunit antibody staining in the hippocampus showed a significant decrease (p < 0.01) in the GABAAα1 receptor subunit in epileptic rat compared to control. Bacopa monnieri, Bacoside-A and Carbamazepine treated epileptic rats showed a significant reversal (p < 0.05) of the decrease in GABAAα1 receptor subunit staining in the hippocampus compared to epileptic rats (Figure 10).
Figure 10.
GABAAα1 receptor subunit antibody staining in the cerebral cortex of Control. and experimental rats. C- Control, E- Epileptic, E+B- Epileptic + Bacopa monnieri, E+D- Epileptic + Bacoside-A, E+C- Epileptic + carbamazepine treated rats. Pixel intensity of experimental rats were C-103465 ± 3043, E- 76456 ± 1593**, E+B-95835@@ ± 2935, E+D-90564 ± 2553@@, E+C-87593 ± 1093@@. () Indicating the GABAAα1 receptor subunit. **p < 0.01 when compared to control, @@p < 0.01 when compared to epileptic group.
Discussion
In this study, we focused on the involvement of cortical GABA receptors in temporal lobe epilepsy, associated mood disorders, spatial learning and memory deficit in epileptic rats. TLE is a common end result of brain-damaging insults with very different etiologies and initial pathologies, such as genetic malformation, head trauma, stroke, infection or status epilepticus [27]. Although there are various reports of the anticonvulsant and neuroprotective properties of Bacopa monnieri and its active component Bacoside-A, little effort has been extended to understand their pharmacological action on the GABA receptors in the cerebral cortex of the epileptic rats. GABA is the major inhibitory neurotransmitter in the central nervous system [28,29]. It exerts an inhibitory action in all forebrain structures and plays a role in the physiopathogenesis of epilepsy [30]. GABAA receptor binding influences the early portion of the GABA mediated inhibitory postsynaptic potential, whereas GABAB binding influences the late portion. GABAA receptor activation in neurons induced a complex physiological response, namely the activation of a Cl- conductance in concert with a blockade of the resting K+ outward conductance results in hyperpolarisation. Both responses were mediated by the activation of GABAA receptors, since they were both mimicked by the GABAA receptor agonist muscimol and antagonized by picrotoxin and bicuculline [2]. Reductions of GABA mediated inhibition and decreased activity of GAD has been reported in studies of human epileptic brain tissue. Impairment of GABA functions produces seizures, whereas enhancement results in an anticonvulsant effect [31,32]. Support for a chronic loss of GABAergic function in epileptogenic human cortex has been based on biochemical assays of tissue resected for relief of focal seizure [33,34]. It is reported that in both epileptic and histologically damaged cortex, there are significant decreases in GAD and GABA binding [33]. We observed a significant decrease in the Bmax of GABA receptors in the cerebral cortex of epileptic rats compared to control GABA receptors and GAD gene expression patterns were similar to the receptor binding studies. Treatment with Bacopa monnieri and Bacoside-A reversed the receptor alterations in Bmax and gene expression to near control. Previous studies from our lab showed that Bacopa monnieri treatment to epileptic rats reduced the number of seizures per hour which is suggestive of its anticonvulsant property [35]. GAD plays a very important role in maintaining excitatory inhibitory balance of the central nervous system [36]. Analysis of GAD activity was used as a marker of over all GABAergic activity and was found to be lower in epileptic mice. The enzymatic activity of GAD is the rate limiting step in the production of GABA and GAD serve as a marker of inhibitory neurons. Moreover, preliminary findings indicate that the decrease in GABA is associated with reduced GABA synthesis rather than increased degradation [37]. In order to provide a direct anatomic analysis of GABAergic neurons and terminals in experimental epilepsy, it is demonstrated a significant decrease in immunocytochemically labeled GAD-positive neurons and puncta in and adjacent to an alumina-damaged epileptic focus in monkey motor cortex [38-40].
There are seizure-dependent increases in CRE-binding activities in various brain regions of the mice [41]. Our study showed that CREB mRNA was significantly decreasing in the cerebral cortex of the epileptic rats. Increased expression of total CREB and phosphorelated CREB found in the human epileptic hippocampus. This when considered with the observation that CREB mediate transcription from the GABAB Receptor1b (GABABR1b) and GABABR1b promoters [42]; epileptic rats exhibit elevated levels of GABA B Receptor 2 (GABABRs) subunit mRNAs and GABABRs antagonist inhibit CREB-DNA binding activity at dose that inhibit seizure behaviour, point to a novel feed back mechanism to GABAB receptor in the generation and propagation of seizure.
The incidence of psychiatric diseases in epileptics is significantly higher than in the general population [43-46]. Depressive and anxiety disorders are the most common psychiatric diseases in this patient group. Recent studies employing magnetic resonance spectroscopy (MRS) suggest that unipolar depression is associated with reductions in cortical GABA levels [47,48]. Antidepressant and mood-stabilizing treatments also appear to raise cortical GABA levels and to ameliorate GABA deficits in patients with mood disorders [49]. Anxiety disorders have long been associated with disturbances of GABA function because of the ability of the benzodiazepine anxiolytics to facilitate brain GABA neurotransmission [50]. Interestingly, as with plasma studies MRS also reveals lowered concentrations of GABA in occipital cortex in panic disorder [51] and in subjects with alcohol dependence [52]. We used two validated psychopharmacological method Y-maze test to quantify depression-like and anxiety-like behavior induced by the decreased GABA receptors in the cerebral cortex of the epileptic rats. Our study showed decreased exploratory behaviour in epileptic rats and treatment using Bacopa monnieri and bacoside-A brings back the exploratory behaviour to near control. This can be correlated with the decreased GABA receptors in the cerebral cortex of the epileptic rat interaction through 5-HT pathways. Immobility in rats is considered to be a state of lowed mood or hopelessness which the rodent experience when they are forced to explores in a constrained space from they cannot escape. There are multiple interactions between central GABA and 5-HT pathways, and some of these interactions provide a theoretical framework in which changes in cortical GABA function can lead to some aspects of disrupted 5-HT function seen in depressed patients [53,54]. In addition, interactions between 5-HT and GABA offer a route by which 5-HT targeted treatments might alter GABA function to bring about their therapeutic effect [55]. Nigel et al., [56] have suggested shared neurobiological processes leading both to seizures and to behavioral, emotional and cognitive disturbance which could possibly explain the how Bacopa monnieri is effective as an anti-convulsant and an anti-depressant. Carbamzepine treatment to epileptic rats also showed a similar effect.
Cerebral cortex plays a key role in memory, attention, perceptual awareness, thought, language, mood and consciousness. Epileptic patients are often suffering from memory and cognitive problems [57-59]. Preliminary studies established that the treatment with crude extract and with the alcoholic extract of Bacopa monnieri plant [60] enhanced learning ability in rats. The radial arm maze experiment was conducted to study the neurobiological mechanisms that underlie spatial learning and memory functions in experimental. Radial arm maze is a tool to examine the neural systems that are involved in memory, and the influence of pharmacological compounds on memory [61]. Radial arm maze experiment demonstrated the impairment in spatial learning and memory in the model studied. The administration of a crude extract of Bacopa monnieri to epileptic rats decreased the trial to attain the criterion performance in the radial arm maze to control levels. These results confirmed the memory enhancing property of Bacopa monnieri in epileptic rats. It is also reported to facilitate the acquisition, consolidation, retention and recall of learned tasks [62] and improve the speed at which visual information is processed. Bacopa monnieri extracts and isolated bacosides have been extensively investigated in several studies for their neuropharmacological effects and a number of reports are available confirming their neuroprotective action [35].
Conclusion
Our experimental results support that decreased GABA receptors and GAD activity in the cerebral cortex comprise an important role in seizure initiation and mood disorders associated with epilepsy. We conclude from our studies that Bacopa monnieri and Bacoside-A treatment potentiates a therapeutic effect by reversing the alterations in general GABA, GABAA, GABAB receptor binding, GABAA receptor subunits, GAD and CREB gene expression that occur during epilepsy, resulting an increased GABA mediated inhibition in the over stimulated cerebral cortex neurons. Bacopa monnieri and Bacoside-A treatment also useful for managing the memory problems and mood disorders associated with the epilepsy. This studies showed the therapeutic significance of Bacopa monnieri, and its active component Bacocide-A in the management of epilepsy, associated mood disorders and memory problems.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JM designed the work and carried out the receptor study. SB participated in the gene amplification studies. SAP and PMA participated in the co focal and behavioural studies. All authors read and approved the final manuscript.
Contributor Information
Jobin Mathew, Email: eattathottu@gmail.com.
Savitha Balakrishnan, Email: lakshmi004in@gmail.com.
Sherin Antony, Email: sherinbinoyg@gmail.com.
Pretty Mary Abraham, Email: prettycusat@gmail.com.
CS Paulose, Email: cspaulose@cusat.ac.in.
Acknowledgements
This work was supported by research grants from DBT, DST, ICMR, Govt. of India and KSCSTE, Govt. of Kerala to Dr C S Paulose. Jobin Mathew thanks CSIR for JRF.
References
- Gregory C, Mathews MD. The Dual Roles of GABA in Seizures and Epilepsy Generate More Excitement. Epilepsy Curr. 2007;7:28–30. doi: 10.1111/j.1535-7511.2007.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrakakis C, Müller T, Schmidt K, Kettenmann H. GABA(A) receptor activation triggers a Cl- conductance increase and a K+ channel blockade in cerebellar granule cells. Neuroscience. 1997;79:177–89. doi: 10.1016/S0306-4522(96)00644-6. [DOI] [PubMed] [Google Scholar]
- Williamson PD, Engel JJ, Munari C. In: Anatomic classification of localization-related epilepsies. Engel JJ Pedley TA, editor. Epilepsy A Comprehensive Textbook. Lippincott-Raven Publishers, Philadelphia; 1997. pp. 2405–2416. [Google Scholar]
- Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia. 1991;32:778–782. doi: 10.1111/j.1528-1157.1991.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Fritschy J. Epilepsy, E/I balance and GABAA receptor plasticity. Front Mol Neurosci. 2008;1:5. doi: 10.3389/neuro.02.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sophie F, Robert FA, Allan J. Tobin, Long term increase of glutamate decarboxylase mRNA in a rat model of temporal lobe epilepsy. Neuron. 1990;5:361–371. doi: 10.1016/0896-6273(90)90172-C. [DOI] [PubMed] [Google Scholar]
- Ishige K, Ito Y, Fukuda H. Cyclic AMP Responsive Element- and Activator Protein 1 DNA-Binding Activities in Epilepsy Model Mice. Journal of the Pharmaceutical Society of Japan. 1999;119:510–518. doi: 10.1248/yakushi1947.119.7_510. [DOI] [PubMed] [Google Scholar]
- Janine L, Steiger SB, David HF, Shelley JR. cAMP Response Element-Binding Protein, Activating Transcription Factor-4, and Upstream Stimulatory Factor Differentially Control Hippocampal GABABR1a and GABABR1b Subunit Gene Expression through Alternative Promoters. The Journal of Neuroscience. 2004;24:6115–6126. doi: 10.1523/JNEUROSCI.1200-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SO. The radial arm maze as a tool in behavioral pharmacology. Physiology & Behavior. 1987;40:793–797. doi: 10.1016/0031-9384(87)90286-1. [DOI] [PubMed] [Google Scholar]
- Caterina M, Hernandez HH, Alvin VT. Spontaneously hypertensive rats: further evaluation of age-related memory performance and cholinergic marker expression. J Psychiatry Neurosci. 2003;28:197–209. [PMC free article] [PubMed] [Google Scholar]
- Murugesan T. Evaluation of psychopharmacological effects of Bacopa monnieri Linn Extract. Phytomedicine. 2005;8:472–476. doi: 10.1078/S0944-7113(04)70068-9. [DOI] [PubMed] [Google Scholar]
- Rosane BB, Helena MT. Carbamazepine enhances discriminative memory in rat model of epilepsy. Epilepsia. 2004;45:1443–7. doi: 10.1111/j.0013-9580.2004.52403.x. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet. 2001;357:216–22. doi: 10.1016/S0140-6736(00)03600-X. [DOI] [PubMed] [Google Scholar]
- Russo A, Izzo AA, Borrelli F, Renis M, Vanella A. Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytother Res. 2003;17:46–54. doi: 10.1002/ptr.1061. [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN. Effect of Bacopa monniera extract on avoidance responses in rat. J Ethnopharmacol. 1982;5:205–14. doi: 10.1016/0378-8741(82)90044-7. [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN. In: Lectures in neurobiology. Tandon PN, Bijiani V, Wadhwa S, editor. Vol. 1. New Delhi:Wiley Eastern; 1992. Drugs affecting learning and memory; pp. 189–207. [Google Scholar]
- Reas SK, Amee K, Paulose CS. Glutamate receptor gene expression and binding studies in pilocarpine induced epileptic rat: neuroprotective role of Bacopa monnieri extract. Epilep Behav. 2008;12:54–60. doi: 10.1016/j.yebeh.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Paulose CS, Chathu F, Khan RS, Krishnakumar A. Neuroprotective role of Bacopa monnieri extract in epilepsy and effect of glucose supplementation during hypoxia: glutamate receptor gene expression. Neurochem Res. 2008;33:1663–1671. doi: 10.1007/s11064-007-9513-8. [DOI] [PubMed] [Google Scholar]
- Krishnakumar A, Nandhu MS, Paulose CS. Upregulation of 5-HT2C receptors in hippocampus of pilocarpine-induced epileptic rats: Antagonism by Bacopa monnieri . Epilepsy and behavior. 2009;16:225–230. doi: 10.1016/j.yebeh.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. After discharge threshold. Electroencephalogr Clin Neurophysiol. 1972;32:269–79. doi: 10.1016/0013-4694(72)90176-9. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Roserbbrough N, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Kurioka S, Toshiaki K, Makoto M. Effect of Sodim and Bicarbonate Ions on GABA Receptor binding in synaptic membrane of rat brain. J Neurochem. 1981;37:418–421. doi: 10.1111/j.1471-4159.1981.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. doi: 10.1111/j.1749-6632.1949.tb27297.x. [DOI] [Google Scholar]
- Ma MX, Chen YM, He J, Zeng T. Effects of morphine and its withdrawal on Y-maze spatial recognition memory in mice. Neuroscience. 2007;147:1059–1065. doi: 10.1016/j.neuroscience.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Akwa Y, Ladurelle N, Covey DF, Baulieu EE. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proceedings of the National Academy of Sciences of the United States of America. 2001;98:14033–14037. doi: 10.1073/pnas.241503698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Pan B, Hawver DB, Wright CB, Potter WZ, Manji HK. Attenuation of cyclic AMP production by carbamazepine. Neurochem. 1996;67:2079–86. doi: 10.1046/j.1471-4159.1996.67052079.x. [DOI] [PubMed] [Google Scholar]
- Engel JJ, Pedley R. In: Epilepsy -A Comprehensive Textbook. Engel JJ, Pedley TA, editor. Lippincott-Raven Publishers; Philadelphia; 1997. pp. 1–10. [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between γ-aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J, Niggemann B, Zaenker KS, Entschladen F. The neurotransmitter γ-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cells. Cancer research. 2002;22:6467–6469. [PubMed] [Google Scholar]
- Smart TG. Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr Opin Neurobiol. 1997;3:358–67. doi: 10.1016/s0959-4388(97)80063-3. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Macdonald RL. Barbiturate enhancement of GABA-mediated inhibition and activation of chloride ion conductance: correlation with anticonvulsant and anesthetic actions. Brain Res. 1981;23:177–88. doi: 10.1016/0006-8993(81)91179-3. [DOI] [PubMed] [Google Scholar]
- MacDonald RL, Barker JL. Enhancement of GABA-mediated postsynaptic inhibition in cultured mammalian spinal cord neurons: a common mode of anticonvulsant action. Brain Res. 1979;167:323–36. doi: 10.1016/0006-8993(79)90826-6. [DOI] [PubMed] [Google Scholar]
- Lloyd KG, Munari C, Bossi L, Stoeffels C, Talairach J, Morselli PL. In: Neuro- transmitters, Seizures, and Epilepsy. Morselli PL, editor. Raven press; 1981. Biochemical evidence for the alterations of GABA- mediated synaptic transmission in pathological brain tissue (stereo EEG or morphological definition) from epileptic patients; pp. 325–338. [Google Scholar]
- Lloyd KG, Bossi L, Morselli PL. In: Neurotransmitters, Seizures, and Epilepsy II. Fariello RG, editor. Raven press; 1984. GABA hypothesis of human epilepsy: Neurochemical evidence from surgically resected identified foci; pp. 285–293. [Google Scholar]
- Reas SK, Amee K, Paulose CS. Decreased glutamate receptor binding and NMDA R1 gene expression in the hippocampus of pilocarpine induced epileptic rats: neuroprotective role of Bacopa monnieri extract. Epilepsy and Behavior. 2008;12:54–60. doi: 10.1016/j.yebeh.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Quan L, Meili G, Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Mason GF, Sanacora G, Hundal R, Petersen K, Shulman G, Graaf R, Rothman D. Preliminary evidence of reduced cortical GABA synthesis rate in major depression. Society for Neuroscience Abstracts. 2001;27:142–6. [Google Scholar]
- Ribak CE, Harris AB, Vaughn JE, Roberts E. Inhibitory GABAergic nerve terminals decrease at sites of focal epilepsy. Science. 1979;205:21l–214. doi: 10.1126/science.205.4401.21. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Hunt CA, Bakay RA, Oertel WH. A decrease in the number of GABAergic somata is associated with the preferential loss of GABAergic terminals at epileptic foci. Brain Res. 1986;363:78–90. doi: 10.1016/0006-8993(86)90660-8. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Harris AB, Vauahn JE, Roberts E. In: Neurotransmitters, Seizures, and Epilepsy. Morselli PL, editor. Raven press; 1981. Immunocytochemical changes in cortical GABA neurons in a monkey model of epilepsy; pp. 11–21. [Google Scholar]
- Ishige K, Ito Y, Fukuda H. Cyclic AMP Responsive Element- and Activator Protein 1 DNA-Binding Activities in Epilepsy Model Mice. Journal of the Pharmaceutical Society of Japan. 1999;119:510–518. doi: 10.1248/yakushi1947.119.7_510. [DOI] [PubMed] [Google Scholar]
- Janine L, Steiger SB, David HF, Shelley JR. cAMP Response Element-Binding Protein, Activating Transcription Factor-4, and Upstream Stimulatory Factor Differentially Control Hippocampal GABABR1a and GABABR1b Subunit Gene Expression through Alternative Promoters. The Journal of Neuroscience. 2004;24:6115–6126. doi: 10.1523/JNEUROSCI.1200-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AM, Soto A. Ictal recordings in postictal psychosis and postictal depression. Neurology. 1998;50:390–397. [Google Scholar]
- Mendez MF, Cummings JL, Benson DF. Depression in epilepsy: significance and phenomenology. Arch Neurol. 1986;43:766–770. doi: 10.1001/archneur.1986.00520080014012. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Doss RC, Taylor JL. Depression in epilepsy: relationship to seizures and anticonvulsant therapy. J Nerv Ment Dis. 1993;181:444–447. doi: 10.1097/00005053-199307000-00007. [DOI] [PubMed] [Google Scholar]
- Robertson MM. In: Ictal and interictal depression in patients with epilepsy, in Aspects of Epilepsy and Psychiatry. Trimble MR, editor. John Wiley & Sons press; 1986. pp. 213–333. [Google Scholar]
- Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, OA Berman RM. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Archives of General Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Critical Reviews in Neurobiology. 2000;14:23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Molecular Psychiatry. 2002;7:71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Honig A, Bartlett JR, Bouras N, Bridges PK. Amino acid levels in depression: a preliminary investigation. Journal of Psychiatric Research. 1988;22:159–164. doi: 10.1016/0022-3956(88)90001-5. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, Charney DS. Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Archives of General Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI. Preliminary evidence of low cortical GABA levels in localized 1H-MR spectra of alcohol-dependent and hepatic encephalopathy patients. American Journal of Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Gellman RL, Aghajanian GK. Pyramidal cells in piriform cortex receive a convergence of inputs from monoamine activated GABAergic interneurons. Brain Research. 1993;600:63–73. doi: 10.1016/0006-8993(93)90402-9. [DOI] [PubMed] [Google Scholar]
- Freund TF, Gulyas AI, Acsady L, Gorcs T, Toth K. Serotonergic control of the hippocampus via local inhibitory interneurons. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:8501–8505. doi: 10.1073/pnas.87.21.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwagar Z, Whale R, Cowen PJ. State and trait abnormalities in serotonin function in major depression. British Journal of Psychiatry. 2002;180:24–28. doi: 10.1192/bjp.180.1.24. [DOI] [PubMed] [Google Scholar]
- Nigel JC, Salzberg MR, Gaurav K, Abbie C, Margaret JM, Terence JO. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Experimen Neurol. 2008;209:254–260. doi: 10.1016/j.expneurol.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Rausch R, Kraemer S, Pietras CJ, Le M, Vickrey BG, Passaro EA. Early and late cognitive changes following temporal lobe surgery for epilepsy. Neurology. 2003;60:951–959. doi: 10.1212/01.wnl.0000048203.23766.a1. [DOI] [PubMed] [Google Scholar]
- Blake RV, Wroe SJ, Breen EK, McCarthy RA. Accelerated forgetting in patients with epilepsy Evidence for an impairment in memory consolidation. Brain. 2000;123:472–483. doi: 10.1093/brain/123.3.472. [DOI] [PubMed] [Google Scholar]
- Butler CR, Zeman AZ. Recent insights into the impairment of memory in epilepsy: transient epileptic amnesia, accelerated long-term forgetting and remote memory impairment. Brain. 2008;131:243–263. doi: 10.1093/brain/awn127. [DOI] [PubMed] [Google Scholar]
- Singh HK, Dhawan BN. Effect of Bacopa monnieri extract on avoidance responses in rat. J Ethnopharmacol. 1982;5:205–214. doi: 10.1016/0378-8741(82)90044-7. [DOI] [PubMed] [Google Scholar]
- Leung LS, Boon KA, Kaibara T, Innis NK. Radial maze per-formance following hippocampal kindling. Behav Brain Res. 1990;40:119–129. doi: 10.1016/0166-4328(90)90004-X. [DOI] [PubMed] [Google Scholar]
- Roodenrys S, Booth D Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacology. 2002;27:279–81. doi: 10.1016/S0893-133X(01)00419-5. [DOI] [PubMed] [Google Scholar]