Abstract
OnabotulinumtoxinA has recently been approved by regulatory agencies in the UK and United States for treatment of chronic migraine based on data generated from the PREEMPT studies. As such, onabotulinumtoxinA is the only prophylactic therapy specifically approved for chronic migraine. Most headache clinicians would agree that acute episodic migraine and chronic migraine differ in their pathophysiology, etiology, diagnosis, and response to pharmacological as well as nonpharmacological therapies. Of the 7 botulinum neurotoxin serotypes, botulinum neurotoxin type A (onabotulinumtoxinA) has been the most thoroughly investigated in preclinical and clinical studies. Based on preclinical studies, onabotulinumtoxinA is known to inhibit the release of excitatory neurotransmitters from both motor and sensory neurons by preventing vesicle fusion to the cell membrane. In addition to the well-documented myorelaxant effects of this neurotoxin, onabotulinumtoxinA can exert a direct analgesic effect that likely involves inhibition of primary and secondary nociceptive neurons. The inhibitory effects of onabotulinumtoxinA are also likely to involve suppressing the activity of myogenic trigger points and decreasing the persistent nociceptive barrage that promotes and maintains central sensitization. This article describes possible mechanisms to explain how onabotulinumtoxinA functions as a therapy for chronic migraine and considers why treatment with the neurotoxin is not effective in some chronic migraineurs.
Keywords: chronic migraine, onabotulinumtoxinA, peripheral sensitization, central sensitization, trigger point, nociception
ONABOTULINUMTOXINA TREATMENT OF CHRONIC MIGRAINE
OnabotulinumtoxinA has recently been approved by regulatory agencies in the UK and United States for treatment of chronic migraine. It is the only prophylactic therapy specifically approved for chronic migraine. The basis for approval were 2 large Phase III randomized, placebo-controlled, parallel clinical trials conducted in North America and Europe entitled Phase III Research Evaluating Migraine Prophylaxis Therapy 1 (PREEMPT 1) and PREEMPT 2.1,2
CHRONIC MIGRAINE – NOT AN EXTENSION OF EPISODIC MIGRAINE
In contrast to the findings of the PREEMPT studies, data from earlier clinical studies demonstrated mixed results for onabotulinumtoxinA in episodic migraine.3-5 Curiously, while commonly used preventive treatments for frequent episodic migraine have demonstrated efficacy when migraine is not yet chronic, these drugs have either not been adequately studied or were reported to have mixed results when studied in populations with chronic migraine.6,7 This suggests that chronic migraine may not simply be an extension of episodic migraine but that there may be central nervous system changes that respond uniquely to different pharmacological interventions as migraine chronifies and transitions from an acute to a persistent pain state.
Most headache clinicians agree that acute episodic migraine and chronic migraine differ in their pathophysiology, etiology, diagnosis, and response to pharmacological as well as nonpharmacological therapies. While acute pain is often described as transient, self-limiting, and serves a protective biological function, chronic pain is not thought to serve a protective function but leads to neuroplastic tissue changes, and becomes detrimental to overall health. Another major difference between episodic and chronic migraine is that while episodic migraine attacks can often be effectively treated, chronic migraine is more refractory or its response is more muted to commonly used antimigraine treatments, including the triptans.8 However, onabotulinumtoxinA, which is not recommended as a preventive treatment for episodic migraine or tension-type headache, is now an approved prophylactic therapy for chronic migraine. An intriguing question to consider is how onabotulinumtoxinA reduces the number of headache days, improves quality of life, and lowers disability scores in patients with chronic migraine.
MECHANISTIC CONSIDERATIONS FOR ONABOTULINUMTOXINA IN CHRONIC MIGRAINE
Neurotoxins obtained from Clostridium botulinum are potent inhibitors of neurotransmission between neurons and muscle, and signaling between neurons.9,10 Of the 7 botulinum neurotoxin serotypes, botulinum neurotoxin type A (onabotulinumtoxinA) has been the most thoroughly investigated in preclinical and clinical studies. OnabotulinumtoxinA functions to inhibit the release of excitatory mediators by preventing the fusion of intracellular vesicles, which contain neurotransmitters, to the cell membrane.11-13 Injection of onabotulinumtoxinA at the designated therapeutic sites in the head, neck, and shoulders would result in internalization of the neurotoxin into nearby motor or sensory neurons and disruption of the soluble N-ethylmaleimide-sensitive factor attachment protein (SNARE) complex that facilitates vesicle fusion and release. Specifically, onabotulinumtoxinA binds and enzymatically cleaves the 25 kDa synaptosomal-associated protein (SNAP-25) that is anchored to the cell membrane and is responsible for binding the vesicle-associated membrane protein (VAMP/synaptobrevin). Thus, internalization of onabotuliunumtoxinA in motor neurons would inhibit the release of acetylcholine, resulting in muscle paralysis. However, internalization of the neurotoxin in sensory neurons that innervate the skin and muscles could potentially inhibit the release of proinflammatory mediators at several sites within the sensory neuron. For example, onabotulinumtoxinA would suppress neurogenic inflammation near the injection site by preventing the release of the neuropeptides calcitonin gene-related peptide (CGRP) and substance P from free nerve endings that provide sensory innervation to the skin and muscles.14,15 In addition, the neurotoxin would exert central effects by blocking the release of CGRP and glutamate from nociceptive nerve fibers terminating in the spinal cord16,17 and, thus, suppress stimulation of second-order neurons and glial cells associated with the maintenance of central sensitization and pain.18-21
Traditionally, onabotulinumtoxinA has been used clinically for the treatment of neuromuscular disorders including focal dystonias and relief of pain associated with cervical and oromandibular dystonias.22 At the cellular level, it is well established that onabotulinumtoxinA blocks the presynaptic release of the neurotransmitter acetylcholine from motor neurons at neuromuscular junctions, and thus can suppress overactivity of specific muscles.9,15,23 Chronic muscle overload and tension in the neck and shoulders can lead to persistent fiber contraction, local ischemia, and the release of proinflammatory mediators, including bradykinin, glutamate, and CGRP, which results in sensitization and activation of primary nociceptors.24,25 Excitation of nociceptive neurons, which can occur from tonic muscle activity (myogenic trigger points), leads to referred pain in the head and face. Referred pain patterns are associated with central hypersensitization and lower pain thresholds of second-order nociceptive neurons associated with the development of central sensitization.26
Interestingly, the sites of onabotulinumtoxinA injections are topographically similar to the myogenic trigger points associated with referred pain locations in the head, neck, and shoulders.27-29 Of clinical significance, muscle pain and tenderness, especially in the shoulders and neck, are physiological symptoms associated with migraine and are more commonly observed as migraine chronifies. Sustained signaling from tonic contraction of craniofacial muscles is sufficient to induce prolonged sensitization of nociceptive neurons.30-32 Furthermore, results from these pre-clinical studies in animals provide evidence that certain cervical spinal cord and trigeminal nociceptive neurons receive nociceptive signals from both the dura and craniofacial muscles. Thus, onabotulinumtoxinA may suppress the activity of myogenic trigger points and decrease the persistent nociceptive barrage that promotes and helps maintain central sensitization. Supporting this notion, results from a recent animal study provide evidence that injection of onabotulinumtoxinA into craniofacial muscles rapidly decreases mechanical sensitivity of temporal muscle nociceptors by inhibiting the central release of glutamate and CGRP from muscle nociceptors.30 In another study, botulinum toxin type A administered subcutaneously or injected intrathecally was found to diminish bilateral hyperalgesia in a model of sustained muscle pain caused by unilateral repeated injections of acidic saline.33 Furthermore, data from a clinical study of abobotulinumtoxinA (Dysport) provided evidence of the antinociceptive effect of injection of botulinum toxin type A in the 10 most tender trigger points in patients with moderate to severe myofacial pain syndrome affecting their cervical and shoulder muscles.34 The percentage of patients reporting mild or no pain was significantly greater in the abobotulinumtoxinA treated group when compared with patients injected with saline. Importantly, muscle tenderness and allodynia have been proposed to be a predictor of responsiveness to onabotulinumtoxinA,35 and therefore, it may be prudent to routinely palpate for trigger points in the neck and shoulder muscles of chronic migraine patients.
Another potential target of onabotulinumtoxinA is directly blocking activity of the trigeminal nerves that provide sensory innervation to the head and face. Results from animal studies have provided evidence that onabotulinumtoxinA can block the stimulated release of CGRP, glutamate, and substance P from trigeminal neurons and inhibit activation of second-order neurons within the spinal cord responsible for transmission of pain signals.14,36,37 In particular, data from inflammatory pain models clearly demonstrate an antinociceptive effect of onabotulinumtoxinA.38-40 Based on these findings, one might assume that the primary therapeutic benefit of using onabotulinumtoxinA for chronic migraine is to repress secretion of inflammatory mediators from trigeminal neurons that mediate the development of peripheral and central sensitization.14,21,40 However, it is difficult to explain at the cellular level how injection of onabotulinumtoxinA in the typical pattern used therapeutically to treat chronic migraine could suppress activation of primary and secondary trigeminal nociceptive neurons directly implicated in migraine pathology. While there is evidence of cross-excitation within the trigeminal ganglia,41-43 there are no reports of cross-inhibition in which suppressing the activity of a subset of neurons in one region of the ganglia leads to decreased activity in other regions. Nevertheless, data from recent studies have provided evidence that the antinociceptive effects of onabotulinumtoxinA may be mediated at the level of the spinal cord.33 In a study by Lackovic and colleagues,44 the antinociceptive effect of botulinum toxin type A was reported to involve axonal transport of the neurotoxin within trigeminal sensory neurons and cleavage of SNAP-25 in nociceptive nuclei in the medullary dorsal horn (spinal trigeminal nucleus). Taken together, data from preclinical studies provide evidence that onabotulinumtoxinA can suppress events associated with peripheral and central sensitization, physiological events implicated in chronic migraine.
While the exact mechanism by which onabotulinumtoxinA functions to reduce the number and severity of headaches in chronic migraineurs is not known, the neurotoxin is likely to function by multiple mechanisms involving inhibition of neurotransmitter release from motor neurons and from sensory nociceptive neurons associated with muscle fibers (Fig. 1). In the proposed model, blocking of acetylcholine release from motor neurons would cause relaxation of overactive muscle fibers and consequently result in a decrease in secretion of inflammatory mediators responsible for sensitization of primary nociceptive neurons. OnabotulinumtoxinA could also function by directly inhibiting the release of proinflammatory mediators from the free endings of peripheral primary nociceptors. If this were to occur, onabotulinumtoxinA would break an inflammatory loop involving activated muscle fibers and nociceptive neurons that promote and maintain peripheral and central sensitization. Finally, based on recent findings,44 the antinociceptive effects of onabotulinumtoxinA are likely to involve suppressing the activation of second-order nociceptive neurons by blocking the release of CGRP and glutamate from primary nociceptors that terminate in the medullary dorsal horn.
Fig 1.
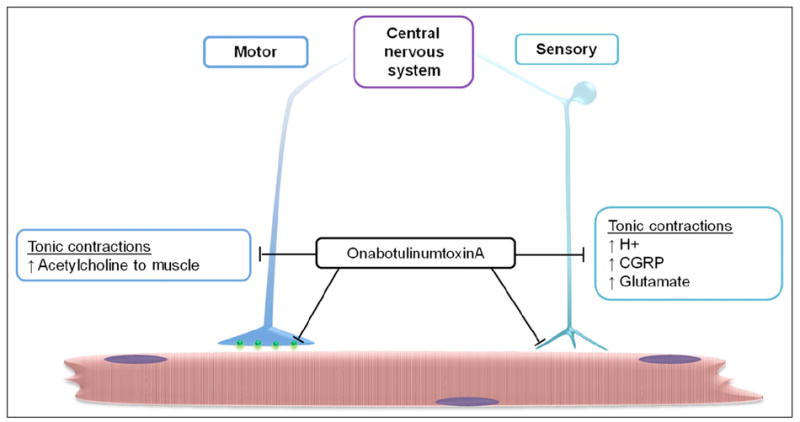
Proposed cellular targets of onabotulinumtoxinA. Injection of neurotoxin in specific sites in the head, neck, and shoulders would result in endocytosis in motor neurons and sensory neurons. Internalization of onabotulinumtoxinA in motor neurons would inhibit release of acetylcholine at the neuromuscular synapse and suppress tonic contractions. OnabotulinumtoxinA would also indirectly repress the release of the proinflammatory mediators including protons (H+), calcitonin gene-related peptide (CGRP), and glutamate. This relase occurs with muscle contraction and is known to promote sensitization and activation of nociceptive neurons. Similarly, onabotulinumtoxinA internalization in sensory neurons would block the release of neuropeptides and other inflammatory mediators that promote peripheral sensitization at the level of the muscles and within trigeminal ganglia. In addition, internalization of the neurotoxin would inhibit the release of proinflammatory mediators at the level of the spinal cord, and thus, suppress activation of second-order nociceptive neurons and glial cells implicated in central sensitization.
An important point to consider is why onabotulinumtoxinA treatment is not effective in all chronic migraine patients. A plausible explanation is that the underlying pathophysiology at the cellular level is not the same in each individual. While it is well established that onabotulinumtoxinA inhibits SNARE-dependent release of neurotransmitters and neuropeptides, there is evidence of calcium- and SNARE-independent mechanisms for secretion of proinflammatory mediators contained in secretory vesicles.45-47 In addition, the ability of onabotulinumtoxinA to block vesicle fusion and neurotransmitter release appears to be dependent on the type of chemical stimulus that causes excitation of the neuron.39,48,49 Furthermore, the release of nitric oxide, which is known to contribute to peripheral and central sensitization of nociceptive neurons, is not inhibited by onabotulinumtoxinA.50 These findings may help to explain why onabotulinumtoxinA is effective in reducing the number of headache days and severity of attack in only a subpopulation of chronic migraineurs.
SUMMARY AND FINAL THOUGHTS
There now exists considerable evidence that supports the notion that onabotulinumtoxinA can exert a direct analgesic effect in addition to its myorelaxant effect. It is likely that the benefit of using onabotulinumtoxinA as a prophylactic treatment for chronic migraine is due to its ability to inhibit overactivity of motor neurons and hyperexcitability of sensory neurons, and involves suppression of peripheral and central sensitization. Given the significant amount of clinical data providing evidence that onabutulinumtoxinA is useful in the management of focal muscle overactivity of cerebral or spinal origin, we predict that onabotulinumtoxinA would be most beneficial in the treatment of chronic migraineurs with active trigger points. In conclusion, it is likely that knowledge gained from future studies of onabotulinumtoxinA and other Clostridium neurotoxins will lead to a better understanding of the underlying mechanisms and more effective treatments for chronic migraine.
Acknowledgments
The authors wish to acknowledge the contributions of Carrie V. Vause for writing and references, Jessica Hall for editing, and Candace Shade for help in preparing the article.
Abbreviations
- CGRP
calcitonin gene-related peptide
- PREEMPT
Phase III Research Evaluating Migraine Prophylaxis Therapy
- SNAP-25
25 kDa synaptosomal-associated protein
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein
- VAMP
vesicle-associated membrane protein
References
- 1.Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 3.Aurora SK, Gawel M, Brandes JL, Pokta S, Vandenburgh AM. Botulinum toxin type A prophylactic treatment of episodic migraine: A randomized, double-blind, placebo-controlled exploratory study. Headache. 2007;47:486–499. doi: 10.1111/j.1526-4610.2006.00624.x. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld A. Botulinum toxin type A as an effective prophylactic treatment in primary headache disorders. Headache. 2003;43:853–860. doi: 10.1046/j.1526-4610.2003.03163.x. [DOI] [PubMed] [Google Scholar]
- 5.Relja M, Poole AC, Schoenen J, Pascual J, Lei X, Thompson C. A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia. 2007;27:492–503. doi: 10.1111/j.1468-2982.2007.01315.x. [DOI] [PubMed] [Google Scholar]
- 6.Silberstein SD, Lipton RB, Saper J. Chronic daily headache. In: Silberstein SD, Lipton RB, Dodick DW, editors. Wolff’s Headache. New York: Oxford University Press, Inc.; 2008. pp. 353–354. [Google Scholar]
- 7.Silberstein SD, Lipton RB, Dodick DW, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: A randomized, double-blind, placebo-controlled trial. Headache. 2007;47:170–180. doi: 10.1111/j.1526-4610.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 8.Schulman EA, Lake AE, 3rd, Goadsby PJ, et al. Defining refractory migraine and refractory chronic migraine: Proposed criteria from the Refractory Headache Special Interest Section of the American Headache Society. Headache. 2008;48:778–782. doi: 10.1111/j.1526-4610.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 9.Pearce LB, First ER, MacCallum RD, Gupta A. Pharmacologic characterization of botulinum toxin for basic science and medicine. Toxicon. 1997;35:1373–1412. doi: 10.1016/s0041-0101(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 10.Schiavo G, Rossetto O, Montecucco C. Clostridial neurotoxins as tools to investigate the molecular events of neurotransmitter release. Semin Cell Biol. 1994;5:221–229. doi: 10.1006/scel.1994.1028. [DOI] [PubMed] [Google Scholar]
- 11.Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427–446. doi: 10.1016/s0300-9084(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 12.Keller JE, Neale EA. The role of the synaptic protein snap-25 in the potency of botulinum neurotoxin type A. J Biol Chem. 2001;276:13476–13482. doi: 10.1074/jbc.M010992200. [DOI] [PubMed] [Google Scholar]
- 13.Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol. 2006;13(Suppl. 4):1–9. doi: 10.1111/j.1468-1331.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 14.Aoki KR. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl. 1):S9–15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- 15.Dolly O. Synaptic transmission: Inhibition of neurotransmitter release by botulinum toxins. Headache. 2003;43(Suppl. 1):S16–S24. doi: 10.1046/j.1526-4610.43.7s.4.x. [DOI] [PubMed] [Google Scholar]
- 16.Sessle BJ. Neural mechanisms and pathways in craniofacial pain. Can J Neurol Sci. 1999;26(Suppl. 3):S7–11. doi: 10.1017/s0317167100000135. [DOI] [PubMed] [Google Scholar]
- 17.Sessle BJ. Acute and chronic craniofacial pain: Brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 18.Watkins LR, Maier SF. Glia: A novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 19.Ren K. Neuron, glia and reciprocal relationships in pain processing. Open Pain J. 2009;2:7–31. doi: 10.2174/1876386301003010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren K. Emerging role of astroglia in pain hypersensitivity. Jpn Dent Sci Rev. 2010;46:86–96. doi: 10.1016/j.jdsr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 22.Evidente VG, Adler CH. An update on the neurologic applications of botulinum toxins. Curr Neurol Neurosci Rep. 2010;10:338–344. doi: 10.1007/s11910-010-0129-z. [DOI] [PubMed] [Google Scholar]
- 23.Bradl M, Lassmann H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 25.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol. 2005;99:1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 26.Graven-Nielsen T, Arendt-Nielsen L. Peripheral and central sensitization in musculoskeletal pain disorders: An experimental approach. Curr Rheumatol Rep. 2002;4:313–321. doi: 10.1007/s11926-002-0040-y. [DOI] [PubMed] [Google Scholar]
- 27.Ford S, Calhoun A, Kahn K, Mann J, Finkel A. Predictors of disability in migraineruus referred to a tertiary clinic: Neck pain, headache characteristics, and coping behaviors. Headache. 2008;48:523–528. doi: 10.1111/j.1526-4610.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-de-las-Penas C, Simons D, Cuadrado ML, Pareja JA. The role of myofascial trigger points in musculoskeletal pain syndromes of the head and neck. Curr Pain Headache Rep. 2007;11:365–372. doi: 10.1007/s11916-007-0219-z. [DOI] [PubMed] [Google Scholar]
- 29.Binderup AT, Arendt-Nielsen L, Madeleine P. Pressure pain sensitivity maps of the neck-shoulder and the low back regions in men and women. BMC Musculoskelet Disord. 2010;11:234–240. doi: 10.1186/1471-2474-11-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam DK, Sessle BJ, Hu JW. Glutamate and capsaicin effects on trigeminal nociception II: Activation and central sensitization in brainstem neurons with deep craniofacial afferent input. Brain Res. 2009;1253:48–59. doi: 10.1016/j.brainres.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 31.Morch CD, Hu JW, Arendt-Nielsen L, Sessle BJ. Convergence of cutaneous, musculoskeletal, dural and visceral afferents onto nociceptive neurons in the first cervical dorsal horn. Eur J Neurosci. 2007;26:142–154. doi: 10.1111/j.1460-9568.2007.05608.x. [DOI] [PubMed] [Google Scholar]
- 32.Amano N, Hu JW, Sessle BJ. Responses of neurons in feline trigeminal subnucleus caudalis (medullary dorsal horn) to cutaneous, intraoral, and muscle afferent stimuli. J Neurophysiol. 1986;55:227–243. doi: 10.1152/jn.1986.55.2.227. [DOI] [PubMed] [Google Scholar]
- 33.Bach-Rojecky L, Lackovic Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol Biochem Behav. 2009;94:234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Gobel H, Heinze A, Reichel G, Hefter H, Benecke R. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: Results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006;125:82–88. doi: 10.1016/j.pain.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Mathew NT. Dynamic optimization of chronic migraine treatment: Current and future options. Neurology. 2009;72:S14–S20. doi: 10.1212/WNL.0b013e3181974821. [DOI] [PubMed] [Google Scholar]
- 36.Foran PG, Mohammed N, Lisk GO, et al. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–1371. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- 37.Durham PL, Cady R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache. 2004;44:35–42. doi: 10.1111/j.1526-4610.2004.04007.x. discussion 42-33. [DOI] [PubMed] [Google Scholar]
- 38.Meng J, Ovsepian SV, Wang J, et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J Neurosci. 2009;29:4981–4992. doi: 10.1523/JNEUROSCI.5490-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng J, Wang J, Lawrence G, Dolly JO. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J Cell Sci. 2007;120:2864–2874. doi: 10.1242/jcs.012211. [DOI] [PubMed] [Google Scholar]
- 40.Aoki KR. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology. 2005;26:785–793. doi: 10.1016/j.neuro.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Damodaram S, Thalakoti S, Freeman SE, Garrett FG, Durham PL. Tonabersat inhibits trigeminal ganglion neuronal-satellite glial cell signaling. Headache. 2009;49:5–20. doi: 10.1111/j.1526-4610.2008.01262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thalakoti S, Patil VV, Damodaram S, et al. Neuron-glia signaling in trigeminal ganglion: Implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman SE, Patil VV, Durham PL. Nitric oxide-proton stimulation of trigeminal ganglion neurons increases mitogen-activated protein kinase and phosphatase expression in neurons and satellite glial cells. Neuroscience. 2008;157:542–555. doi: 10.1016/j.neuroscience.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matak I, Bach-Rojecky L, Filipovic B, Lackovic Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Searl TJ, Silinsky EM. Modulation of calcium-dependent and -independent acetylcholine release from motor nerve endings. J Mol Neurosci. 2006;30:215–218. doi: 10.1385/JMN:30:1:215. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C, Zhou Z. Ca(2+)-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci. 2002;5:425–430. doi: 10.1038/nn845. [DOI] [PubMed] [Google Scholar]
- 47.Zheng H, Fan J, Xiong W, et al. Action potential modulates Ca2+-dependent and Ca2+-independent secretion in a sensory neuron. Biophys J. 2009;96:2449–2456. doi: 10.1016/j.bpj.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trudeau L, Fang Y, Haydon PG. Modulation of an early step in the secretory machinery in hippocampal nerve terminals. Proc Natl Acad Sci U S A. 1998;95:7163–7168. doi: 10.1073/pnas.95.12.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bronk P, Deak F, Wilson MC, Liu X, Sudhof TC, Kavalali ET. Differential effects of SNAP-25 deletion on Ca2+-dependent and Ca2+-independent neurotransmission. J Neurophysiol. 2007;98:794–806. doi: 10.1152/jn.00226.2007. [DOI] [PubMed] [Google Scholar]
- 50.Morris JL, Jobling P, Gibbins IL. Differential inhibition by botulinum neurotoxin A of cotransmitters released from autonomic vasodilator neurons. Am J Physiol Heart Circ Physiol. 2001;281:H2124–H2132. doi: 10.1152/ajpheart.2001.281.5.H2124. [DOI] [PubMed] [Google Scholar]


