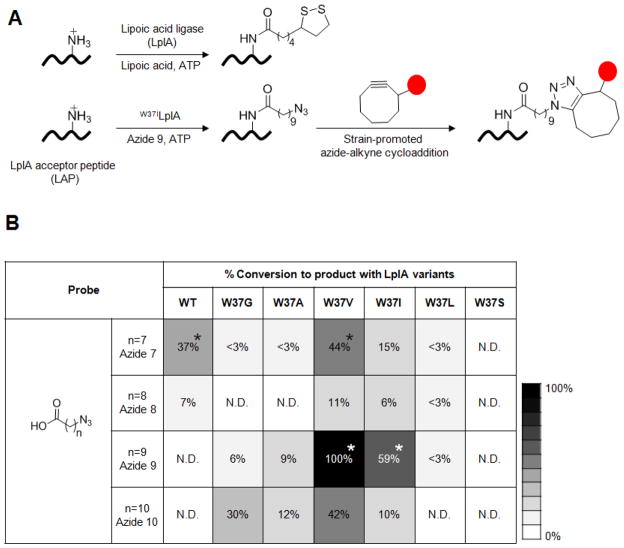
Figure 1.
Fluorophore targeting via LplA-catalyzed azide ligation followed by strain-promoted azide-alkyne cycloaddition. (A) Top: natural ligation of lipoic acid catalyzed by wild-type LplA.12 Bottom: two-step fluorophore targeting used in this work. First, the W37ILplA mutant ligates 10-azidodecanoic acid (“azide 9”) onto the 13-amino acid LplA acceptor peptide (LAP).13 Second, the azido moiety is chemoselectively derivatized using a cyclooctyne-fluorophore conjugate, via strain-promoted, copper-free [3+2] cycloaddition.24 The red circle represents any fluorophore or probe. (B) Screening to identify the best LplA mutant/azide substrate pair. The table shows relative conversions (normalized to that of the W37VLplA/azide 9 pair, which is set to 100%) of LAP to the LAP-azide product conjugate. Wild-type LplA and six W37 point mutants were screened against four azidoalkanoic acid substrates of various lengths. N.D. indicates that product was not detected. Screening was performed with 100 nM ligase, 600 μM LAP and 20 μM azide substrate for 20 min at 30 °C. Conversions were measured in duplicate. Note that W37SLplA was active with the natural substrate, lipoic acid (data not shown), despite being inactive with all the azide substrates. The starred combinations in the table were evaluated and compared in Figure 2.