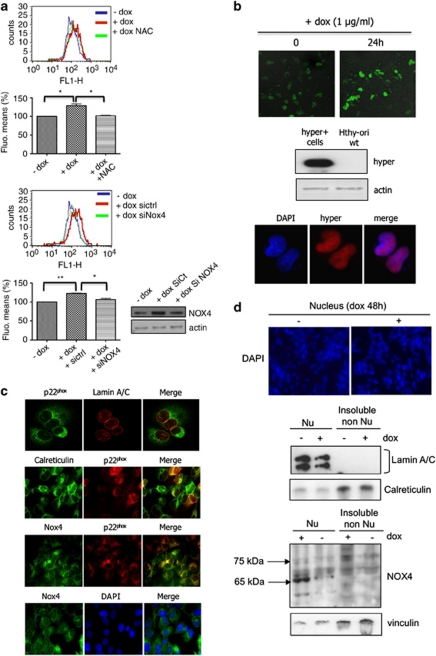
Figure 3.
H-RasV12 increases levels of intracellular ROS via NOX4 activity. (a) The intracellular level of ROS was measured in Tet-on-regulated H-RasV12 HThy-ori cells by DCF fluorescence using flow cytometry. Before treatment with doxycycline (1 μg/ml) for 48 h, cells were transduced with short interfering RNAs to NOX4 or treated for 30 min with 5 m NAC. Graphs show the quantification of fluorescence mean. Immunoblot detection of NOX4 in HThy-ori cells transduced with siRNA control and siRNA NOX4 SMARTpool, as control. Values are the means±s.e. from independent experiments, *P<0.05, **P<0.01. (b) HyPer response to H-RasV12 induction. Confocal analysis of H2O2-dependent fluorescence in Tet-on-regulated H-RasV12 HThy-ori cells-expressing pHyPer-nuc. Magnification × 20. Western blot and immunofluorescence analysis of Hyper-nuc transduced in H-RasV12-inducible cells using anti-green fluorescent protein. Magnification × 63. (c) Colocalization between nucleus membrane protein (laminA/C), endoplasmic reticulum marker (calreticulin), NOX4 and p22phox by immunofluorescence in cells transduced with H-RasV12-expressing vector. Magnification × 63. The nuclei were stained with DAPI (4',6-diamidino-2-phenylindole; blue). (d) Immunoblot detection of NOX4 protein in nuclear fraction (500g pellet, Nu) and insoluble non-nuclear fraction (200 000g pellet, non-Nu) of Tet-on-regulated H-RasV12 HThy-ori cells treated with or without doxycycline (1 μg/ml) for 48 h. Nucleus were stained with DAPI and visualized on microscopy. Magnification × 20. The distribution of NOX4 in non-nuclear and nuclear fractions was characterized using antibodies against fraction-specific proteins: lamin A/C for nuclear fraction and calreticulin for reticulum-enriched insoluble non-nuclear fraction.