Abstract
Human reproduction has benefited significantly by investigating non-human primate (NHP) models, especially rhesus macaques. To expand the Old World monkey species available for human reproductive studies, we present protocols in baboons, our closest Old World primate relatives, for Assisted Reproductive Technologies (ART) leading to live-born offspring. Baboons complement rhesus by confirming or modifying observations generated in humans often obtained by the study of clinically-discarded specimens donated by anonymous infertility patient-couples. Here, baboon ART protocols, including oocyte collection, in vitro fertilization, intracytoplasmic sperm injection (ICSI), preimplantation development to blastocyst stage and embryo transfer techniques are described. With baboon ART methodologies in place, motility during baboon fertilization was investigated by time-lapse video microscopy. The first ART baboons produced by ICSI, a pair of male twins, were delivered naturally at 165 days post-gestation. Genetic testing of these twins confirmed their ART parental origins and demonstrated that they are unrelated fraternal twins, not identicals. These results have implications for ART outcomes, embryonic stem cell derivation, and reproductive sciences.
Keywords: baboon, ART, in vitro fertilization, blastocyst, embryo transfer
Introduction
Understanding the process of fertilization in humans is vital for the diagnosis and treatment of clinical infertility and birth defects (Basatemur and Sutcliffe, 2008), as well as for discovering our reproductive origins. In addition, the emergence of regenerative medicine and its reliance on pluripotent stem cells, especially embryonic stem cells, makes it crucial to learn detailed mechanistic information about nuclear and extranuclear contributions from each gamete to the zygote and embryo. Since the development of human ART (Edwards et al.; Steptoe and Edwards; Steptoe and Edwards; Steptoe et al.), clinically discarded specimens have been donated by anonymous infertile patient-couples for investigations on the process of human fertilization (Simerly et al.; Van Blerkom). While these studies have generated important knowledge, questions remain regarding the normalcy of the human material investigated, since it was developmentally compromised and anonymously donated. To address those concerns, and to verify accuracy, nonhuman primate studies using Old World macaques were conducted (reviewed by (Bavister; Wolf)). From an evolutionary perspective, baboons are closer to humans than macaques (Rogers et al.; Stewart and Disotell) and are readily available as a pedigreed research resource that mirrors many complex disease traits found in humans (Chai et al.; Cox et al.; D'Hooghe et al.; D'Hooghe et al.; D'Hooghe et al.; Fazleabas; Fazleabas and Strakova; Havill et al.; Havill et al.; Hlusko et al.; Inder et al.; Redl and Bahrami; Rietzler et al.; Rogers and Hixson; Tejero et al.; VandeBerg et al.; Vinson et al.).
Assisted Reproductive Technologies (ART) using nonhuman primates (NHPs) have been instrumental in bridging gaps between research in rodents, nearly exclusively using mice, and humans (reviewed by ((Hewitson, 2004); (Nyachieo et al., 2009a 2009c; Nyachieo et al., 2009b 2009c; Nyachieo et al., 2009c 2009c). Now thirty years after the birth of the first child conceived by ART (Edwards et al.; Steptoe and Edwards; Steptoe and Edwards; Steptoe et al.), clinical ART practices raise significant biological and medical issues – many of which can best be solved by responsible investigations of NHP ART outcomes. Questions remain regarding the normalcy of epigenetic imprints after ART (Kobayashi et al.; Li et al.; Lim et al.) and NHPs with gametes that display informative polymorphisms in imprinted domains could resolve debates over whether clinical extrapolations of imprinting losses during mouse ART (Rivera et al.) are reasonable. The precise manner in which mouse embryonic axis and body pattern determination are specified is still a matter of lively debate (Bischoff et al.; Gardner; Kloc et al.; Motosugi et al.; Plusa et al.; Plusa et al.). Because the embryologist determines the site of sperm introduction during ICSI, questions can be posed as to whether the specification of embryonic axes after ICSI might be inadvertently manipulated with perhaps unforeseen consequences; again investigations with NHPs can provide solutions. Mitochondrial inheritance (St John et al., 2005) and other epigenomic regulators, including microRNAs (Yu et al., 2005), cannot be definitively investigated using discarded clinical materials, which are donated anonymously and therefore stripped of all crucial pedigree information. Round spermatid injections routinely result in conception in mice (Kimura and Yanagimachi; Ogura et al.), motivating extrapolations to humans (Khalili et al., 2002). NHP studies with spermatids at varying stages demonstrated that only elongated spermatids succeeded (Hewitson et al.), highlighting differences between rodents and primates.
While in vitro fertilization (IVF) has progressed enormously during the past thirty years of ART, the implantation and maintenance of pregnancies to term remain urgent as research topics in order to improve the efficiencies of ART and reduce multiple pregnancies (reviewed by (Elster; Gerris and Van Royen; Khalaf et al.)). Increasingly, high resolution MRI and PET detection of maternal-fetal dialogues during pregnancy, which can now be non-invasively studied using NHPs, are helping advance these fields significantly (Ahrens et al., 2006). The final rationale for introducing the baboon model involves the burgeoning field of pluripotent stem cell (PSC) research, with its origins in embryonic stem cell (ESC) and germ cell studies, which continues to rely on NHP breakthroughs to set the stage for parallel achievements in humans. NHP ESC lines were established first (Thomson et al., 1995) and human ESCs only described a few years later (Thomson et al., 1998). Similarly, parthenogenetic ESCs were first characterized in NHPs (Cibelli et al.) and several years later in humans (Lin et al.; Mai et al.; Revazova et al.). More recently, ESCs were derived from NHP blastocysts generated by somatic cell nuclear transfer (Byrne et al., 2007) and clinically-oriented teams are continuing this research using human (French et al.; Stojkovic et al.) or hybrid oocytes (Bowles et al.).
There are many compelling biological and medical rationales for preclinical studies using NHPs. Ironically, however, NHP ART work has remained surprisingly specific – nearly all research is performed with macaques, mostly using rhesus, ideally of Indian origin. This is not unlike rodent ART investigations, which mainly involve laboratory mice and often from inbred strains; indeed mouse ESCs have been available for decades, whereas rat ESCs have only just been generated using unconventional approaches (Brons et al.; Iannaccone and Jacob; Li et al.). Because research advances by confirming results in related species, and also because variations exist between related species, we have sought to expand the baboon as an alternative research resource to macaques (review by (Nyachieo et al., 2009a; Nyachieo et al., 2009b; Nyachieo et al., 2009c)). Previously, Nyachieo et al. (2009) had shown that the baboon menstrual cycle could be synchronized using oral contraceptives followed by successful ovarian stimulation using a GnRH agonist in combination with human recombinant hormones. The authors showed an average yield of 19 oocytes at retrievals, with an ICSI fertilization rate of 23-54% and successful development to the 8-cell stage. No pregnancies were produced after embryo transfer, however.
It is noteworthy that, aside from the apes (in which ART research is nearly impossible now), baboons are the most closely related primate species to humans (Banaszak et al.; Chai et al.; D'Hooghe et al.; Fazleabas; Shaikh et al.; Shearer et al.). Also, baboons, unlike macaques, are much more readily available and their genetics, physiology, endocrinology, anatomy, and complex disease phenotypes render them as another crucial and, as of yet, underappreciated research resource. Furthermore, the female's prominent sex skin serves as a reliable non-invasive bioassay for pregnancy. In order to enrich the NHP research resources available for ART, developmental biology, stem cells and regenerative medicine, we present here our methods for baboon ART. These include animal husbandry, ovarian stimulation, sperm collection protocols, in vitro fertilization, including intracytoplasmic sperm injection, preimplantation development conditions, embryo transfer procedures, and management strategies for pregnancies.
Materials and Methods
Baboon Selection Criteria
All nonhuman primate experiments were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals (National Academy of Science, copyright 1996) and approved by institutional IACUC committees at the University of Pittsburgh (#0802725-2) and Southwest Foundation for Biomedical Research (#986 PC). Ninety-six female Baboons (Papio anubis) of breeding age (> 5 yrs; average age= 7.6 yrs; range: 5.0 yrs to 16.6 yrs) were selected for hormonal stimulation and an additional 26 females of proven fertility were selected for embryo transfer after passing a series of health checks (general physical examination, complete blood count (CBC), chemistry panel, palpation of reproductive structures). A daily cycle reading was performed for a minimum of two months to ensure stable, regular menstrual cycles in females (average cycle length= 33.5 ± 0.5 days; n=91; (Shaikh et al., 1982)). Perineal sex skin changes permitted a convenient visual monitoring system to determine female cycle stage (menses onset; ovulation time) without having to resort to daily animal sedation for invasive blood draws and ELISA hormonal assays. As extensively reported by others, we rated the sex skin turgescence via a daily morning visual reading of each female (group-housed or individually-caged) and assigned a scale reading from 0 to 4, with a value of 4 equated to maximum turgescence (Hendrickx and Kraemer, 1968; Pope et al., 1982; Shaikh et al., 1982; Stevens, 1997; Zuckerman and Parkes, 1932). Menses onset generally occurred at the end of minimum turgescence (value=0) and predicted ovulation determined by counting back 2 days from deturgescence.
Colony Management and Husbandry
Unlike smaller macaques, which can be transferred from cage to cage as individuals, baboon husbandry benefits from group housing for the maintenance of group dynamics. In addition to individual caging of selected female baboons for time periods of < 6 months, we also explored whether ovarian stimulation by daily injection was possible while in group housing settings. Harems of 12-17 female baboons were housed with a vasectomized male and their cycles monitored by observing both perineal sex skin, as previously described (Shaikh et al., 1982), and menstrual bleeding. Females at the correct stage of their cycle, as determined by menstrual bleeding, were moved through sectioned chutes containing squeeze cages for daily hormone administration by intramuscular or subcutaneous injections. Twelve hours before scheduled surgery, females were isolated in individual chute partitions and food withdrawn prior to anesthesia administration.
Ovarian Stimulation Procedures
We investigated 9 protocols for the hyperstimulation of female baboons (Table 1), starting on Days 1-2 from the onset of visible menses (Nyachieo et al., 2009a; Nyachieo et al., 2009b; Nyachieo et al., 2009c). Protocol I: a single daily intr-amuscular injection of 60 IU recombinant human follicle stimulating hormone (r-FSH; Gonal-F; Serono, Randolph, MA) for 9 days, followed by a final subcutaneous injection of 1000 IU of recombinant human chorionic gonadotropin (r-hCG; Ovidrel; Serono); Protocol II: a daily intra-muscular injection of 60 IU r-FSH and daily subcutaneous injection of 0.25 μg/ml of a gonadotropin-releasing hormone (GnRH) agonist (Leuprolide Acetate; Sicor Pharmaceuticals, Irvine, CA) for 9 days, the final 3 of which also included 60 IU recombinant human luteinizing hormone (r-hLH; Luveris; Serono) combined with the r-FSH. A final subcutaneous injection of 1000 IU of r-hCG was given to induce oocyte maturation; Protocol III: same as protocol I, except the r-hCG dose was increased to 2500 IU; Protocol IV: same a protocol II, but without the agonist and increasing the dose of r-hCG to 2500 IU; Protocol V: a single, daily, combined intramuscular injection of 60 IU r-FSH and 60 IU recombinant human luteinizing hormone (rhLH; Luveris; Serono) for 9 days, followed by 2500 IU r-hCG administered subcutaneously on Day 10; Protocol VI: same as protocol V, but with 9 days of agonist administration and an increased dosage of r-hCG to 5000 IU; Protocol VII: an extended hormonal stimulation protocol in which the daily injection containing 60 IU r-FSH and 60 IU r-LH varied between 10-13 days, followed by a single subcutaneous injection of 2500 IU r-hCG; this was performed to investigate if extending hormonal stimulation produced superior quantity and quality oocytes, given the longer menstrual cycle observed in the baboon. Protocol VIII: a combined shot of 60 IU r-FSH and 60 IU r-LH for 13 days with an increase of r-hCG to 5000 IU; and Protocol VIX: same as protocol VII but with an increased dose of r-hCG to 5000 IU. Hormones were administered either subcutaneously or intramuscularly according to the manufacturer's instructions and all hormones were used within the specified expiration date. Of the 96 females employed in this study, 14 were stimulated twice with minimal refractoriness to recombinant hormones observed. Consequently, all results were pooled. For all protocols, ultrasonography (Siemens Sonoline Antares; Model 5936518; Siemens Medical Systems, Inc, Issaquah, WA) was performed on the day of surgery to confirm that 3-4 follicles of >3-4mm were present (Bavister et al., 1983), indicating that stimulation was on-going and met the conditions of our approved Institutional Animal Care and Use Committee (IACUC) protocols to perform laparoscopy. Mature follicles were aspirated by laparoscopy 30-32 hours post-hCG (Wolf, 2004) using a Cook® A.R.T. Echotip® Ovum Aspiration Needle (Cook Ob/Gyn; Spencer, IN) attached to a vacuum aspiration pump (Cook Aspiration Unit; Model K-MAR-5100; Cook Medical, Inc, Bloomingdale, IN) and set to a constant pressure of 90mm Hg (Bavister et al., 1983). Multiple individual follicles were aspirated into sterile collection tubes containing modified Tyrodes culture medium with bovine serum albumin, sodium lactate and sodium pyruvate - 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (TALP-HEPES) medium supplemented with heparin and then transported to a dedicated primate ART laboratory (≤ 5 min) for oocyte recovery and evaluation.
Table 1.
Baboon Hormone Stimulation Protocols: Average Oocyte Stages at Collection and Expanded Blastocyst Rates
| [a] Protocol # | [b] # Days of 60 IU r-FSH | [c] # Days of 60 IU r-LH | [d] GnRH Agonist (9 days) | [e] hCG dose (IU) | [f] Collection Day | [g] # of Stim Trials1 | Average oocytes by stage at collection | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [h] GV (Mean ± SD) | [i] GVBD Mean ± SD) | [j] PB1 (Mean ± SD) | [k] Total (Mean ± SD | [l] Avg # of Oocytes Fertilized (Mean ± SD)2 [% of Total] | [m] Ex Blasts Rate (%) | |||||||
| I | 9 | 0 | - | 1000 | 11 | 7 | 25.3 ± 18.9 | 5.4 ± 4.0 | 1.4 ± 1.6 | 32.7 ± 21.3 | 8.7 ± 7.6a [27] | 0/64 (0) |
| II | 9 | 3 | + | 1000 | 11 | 7 | 21.3 ± 12.9 | 3.7 ± 4.8 | 2.6 ± 2.6 | 29.4 ± 11.5 | 6.0 ± 6.5a (20) | 2/42 (5) |
| III | 9 | 0 | - | 2500 | 11 | 5 | 33.0 ± 33.0 | 9.6 ± 11.1 | 1.4 ± 1.1 | 48.6 ± 33.6 | 12.8 ± 12.3b [26] | 0/61 (0) |
| IV | 9 | 3 | - | 2500 | 11 | 20 | 32.8 ± 21.2 | 7.5 ± 7.7 | 8.2 ± 8.3 | 49.4 ± 30.2 | 16.9 ± 13.9b [34] | 2/338 (1) |
| V | 9 | 9 | - | 2500 | 11 | 16 | 24.3 ± 16.0 | 5.7 ± 5.1 | 4.4 ± 4.4 | 34.6 ± 20.9 | 11.6 ± 9.3b [34] | 11/185 (6) |
| VI | 9 | 9 | + | 5000 | 11 | 8 | 39.4 ± 31.6 | 12.5 ± 7.1 | 14.6 ± 7.4 | 66.7 ± 30.5 | 28.6 ± 13.4c (43) | 3/229 (1) |
| VII | 10-13 | 10-13 | - | 2500 | 12-15 | 15 | 28.0 ± 31.7 | 5.5 ± 5.9 | 8.4 ± 7.0 | 41.9 ± 28.9 | 13.7 ± 9.1b (33) | 3/205 (3) |
| VIII | 13 | 13 | - | 5000 | 15 | 7 | 27.6 ± 23.5 | 3.3 ± 5.0 | 10.4 ± 13.3 | 42.4 ± 36.4 | 14.9 ± 15.9b (35) | 1/104 (1) |
| VIX | 10-13 | 10-13 | + | 5000 | 12-15 | 11 | 20.7 ± 15.9 | 4.1 ± 4.1 | 9.2 ± 7.8 | 37.2 ± 22.7 | 13.5 ± 9.4b (36) | 0/148 (0) |
number of stimulation trial attempts
means followed by the same letter do not differ significantly from one another (p≤ 0.05)
Oocyte Collection and Maturation In Vitro
Aspirates were diluted in 10 ml of warm TALP-HEPES medium supplemented with 2.0 mg/ml hyaluronidase (Sigma, St. Louis, MO) (Bavister et al., 1983) and passed through an EM Con™ filter (Immuno Systems, Inc., Spring Valley, WI). Adhering cumulus cells were stripped from oocytes with a mechanical pipette (Mid-Atlantic Diagnostics, Mount Laurel, NJ), quickly sorted according to maturational stage, and placed into TALP media (37°C/5% CO2).
Sperm Collection
Fresh semen from mature male baboons (n=10; weight range: 21-35kg; age range: 9-18 years) was collected on the day of oocyte collection using rectal probe ejaculation (P-T Electronics, Boring, OR; Model # PET 110 VAC) under approved institutional standard operating procedures (SOP No: SNRPC 485.01). The liquid fraction was separated from the coagulant and motile sperm were isolated using a PureCeption™ gradient (Sage In-Vitro Fertilization, Inc, Trumbull, CT) according to manufacturer's instructions. Typical concentrations of sperm harvested after the gradient procedure were between 20-90 × 10-6 sperm per milliter, with greater than 70% forward progressive motility and with less than 10% abnormal forms (bent tails; abnormal head structures; etc). Motile sperm were washed once in TALP-HEPES (Hewitson et al.) by centrifugation (200x g; 8 minutes) and the sperm re-suspended into 500 μl of a fresh media kept at room temperature. . Sperm were used for inseminations within 4-6 hours post-ejaculation.
Intracytoplasmic Sperm Injection
Embryos were conceived from fertile baboons using intracytoplasmic sperm injection (ICSI) and developed to the blastocyst stage (Hewitson et al., 1998). Washed sperm were diluted into sperm handling media containing polyvinylpyrrolidone (PVP) to reduce motility (Irvine Scientific, Irvine, CA). A single motile sperm of normal morphology was aspirated tail-first into a 6-7μm beveled micropipette (Humagen, Charlottesville, VA) and injected into mature oocytes, avoiding the first polar body. After ICSI, injected oocytes were cultured individually in 25μl media droplets under sterile mineral oil in TALP for 12-16 hours before confirming fertilization. Successful fertilization was defined as zygotes with 2 polar bodies and the presence of 2 pronuclei.
Embryo Culture
After the first division, two-cell embryos produced in TALP media were individually transferred to 100 μl droplets of CMRL-1066 media containing 10% fetal calf serum (Hyclone Laboratories, Inc, Logan, UT) with monolayers of buffalo rat liver cells (BRL 1442; American Type Culture Collection, Rockville, MD) at 37°C in 5% CO2 (Hewitson et al., 1998). Embryonic development was scored daily, with changes to fresh CMRL + BRL co-culture plates performed every 2 days until expanded blastocysts were achieved.
Assaying Embryonic Development
Ranked and random growing baboon embryos were analyzed for developmental efficiency in the following ways: i. In vitro preimplantation development to the blastocyst stage; ii. Time-lapse video microscopy; iv. Embryonic stem cell derivations by inner cell mass (ICM) isolations; and v. Embryo transfers for the purposes of establishing ART pregnancies.
Time-lapse Video Microscopy (TLVM)
Forty-one ICSI fertilized baboon oocytes were imaged by TLVM to observe pronuclear formation, apposition, and cytoplasmic positioning, as well as cleavages for up to 48 hours. Following ICSI, an oocyte was placed into a 35mm sterile glass bottom dish (Matec, Inc, Ashland, MA) containing pre-gassed TALP culture medium and immediately placed into a Tokai HIT incubation system (INU system; Tokai, Shizuoka-ken, Japan) on a Nikon TE-300 inverted microscope equipped with Hoffman Modulation Contrast optics. A Plexiglas box with a secondary heater enclosed the entire microscope stage and objectives to ensure accurate control of gas and temperature parameters (5% CO2 / 37°C). Image capture was typically every 10 minutes and accomplished using MetaMorph™ software (Molecular Devices, Sunnyvale, CA). A z-stepper motor (MFC-2000; ASI, Inc, Eugene, OR) was used to collect 5-μm optical sections and a Uniblitz shutter (Vincent Associates, Rochester, NY) employed to limit halogen light exposure after completing a z-stack collection. Both were controlled by the Metamorph™ software.
Embryonic Stem Cell Derivations by Inner Cell Mass (ICM) Isolations has been described recently (Simerly et al., 2009).
Embryo Transfers
Baboon females with at least three months of normal menstrual cycles were selected as embryo recipients. Since each recipient females skin sex is read daily, we were able to select recipients with less than 1-2 days of variability from the predicted day of ovulation, as determined by time of sex skin deturgescence (Shaikh et al., 1982). Females with large variations in their cycles were excluded as embryo recipients. Heterologous embryo transfers were performed over a range of days after predicted ovulation (Table 3). ETs performed 1-3 days post ovulation, but before sex skin drop, were accurately staged only after the recipient underwent deturgescence. However, we found our intra-animal accuracy was within 1-2 days of predictions. For synchrony of embryo stages with the recipient, we attempted to use fertilized- to -early cleavage stage embryos with recipients staged between days 1-4 post ovulation. Late stage preimplantation embryos were transferred into Day 5-7 post ovulation recipients, as based on when flushed morula and blastocysts were retrieved from naturally mated baboons (Pope et al., 1982). Embryo transfers were accomplished by either a mid-ventral, laparoscopic or non-surgical intrauterine transfer. We chose heterologous embryo transfers since preliminary data indicated that homologous embryo transfers, where embryos are returned to the same female from which the oocytes were collected, did not succeed (n=12; our unpublished observations). Perhaps high levels of circulating hCG from exogenous hormone stimulation may interfere with homologous embryo transfers success. For the mid-ventral, laparoscopic embryo transfer, the oviduct was cannulated and up to five 4-cell-to-morula stage ICSI embryos were transferred by a small catheter (Patton Laparoscopic Gift Catheter Introducer Set; Cook® Ob/Gyn; Spencer, IN; (Hewitson et al., 1999)). For intrauterine embryo transfers, the cervix was viewed with a vaginal speculum and the external cervical was swabbed with sterile gauze prior to being cannulated (Patton Laparoscopic Gift Catheter Introducer Set; Cook® Ob/Gyn; Spencer, IN). A small catheter with up to five morula-to-blastocyst stage embryos was passed through the cannula and into the uterine lumen, with embryos being deposited near the uterine fundus (Pope et al., 1982). Implantation was assessed by visually monitoring sex skin tumescence for pregnancy color changes or until the start of menses, indicating no pregnancy establishment. Blinded DNA samples of the ART twins, their parents as well as unrelated adults were analyzed by the Primate Pedigree Laboratory at the California National Primate Research Center at UC-Davis by Dr. C. Penedo.
Table 3.
Embryo Transfer Results in the Baboon.
| Embryo Transfer Type | Recipient Stage (Day 1 = Ov day)1 | Recipient Perineal Stage (range)2 | Total # Embryos Transferred (# of recipients) | Embryonic Developmental Stage at Transfer3 | PC Calls4 | Outcome5 |
|---|---|---|---|---|---|---|
| Laparoscopic | 1 | 4 | 6 (2) | (2) 1-cell, (4) 2PN/Mitotic | 1 | NP |
| Laparoscopic | 2 | 4 | 12 (3) | (1) 8C, (10) 16-32C, (1) Morula | 2 | NP |
| Laparoscopic | 3 | 4 | 27 (7) | (11) 1-cells; (3) 2PN, (2) 6C, (3) 8C, (8) 16-32C | 4 | 1-Preg5 (Twins born 07 Dec 09) |
| Laparoscopic | 4 | (2-3) | 7 (2) | (2) 6C, (5) 16-32C | 1 | NP |
| Laparoscopic | 5 | (1-2) | 20 (6) | (3) 8C, (1) 16C, (5) Ebl, (10) Morula, (1) CMorula | 2 | NP |
| Laparoscopic | 6 | 1 | 3 (1) | (3) 16C | 1 | NP |
| Transcervical | 2 | 4 | 5 (1) | (1) Exbl, (4) Morula | 0 | NP |
| Transcervical | 5 | (1-2) | 13 (3) | (9) Ebl, (1) Exbl, (3) Morula | 0 | NP |
| Transcervical | 7 | 0 | 8 (1) | (8) 16-32C | 1 | Aborted oDay 12 |
| Summary of ET Data for Baboons. | |||||
|---|---|---|---|---|---|
| Transfer Type | Total # trials done | Total # Embryos | Total # Called with Pregnant Color (% of total trials) | Total # with Implantation (% of PC calls) | # Confirmed Heartbeat or Live Birth |
| Laparoscopic | 21 | 75 | 11 (52) | 1 (9) | 1 (Twins) |
| Transcervical | 5 | 26 | 1 (20) | 1 (100) | 0 |
Ovulation estimated as the third day preceding sex skin detumescence.
Scale= 0-4, with 4 maximum turgescence.
PN: pronuclear stage; Ebl: early blastocyst; Exbl: expanded blastocyst; Cmorula: compacted morula. Only a single clutch of donor oocytes were transferred to each recipient.
Demonstrated pregnancy color of sex skin.
As determined by return to normal menses.
6Gamete intrafollicular transfer pregnancy, at Day 32 post-ET
Statistics
We applied the one-way Analysis of Variance (ANOVA) test and compared the means ± standard deviations for the various stimulation protocols using the Multiple Range Test. Mean values reported in Table 1 and followed by the same letter do not differ significantly from one another (p≤ 0.05).
Results
Ovarian Stimulation and Oocyte Collection
Table 1 summarizes the results of hormonal stimulation in baboons using the 9 protocols outlined in the Methods section. At collection, we recorded the number of immature (Table 1, germinal vesicle [GV] stage, column h), maturing (Table 1, germinal vesicle breakdown [GVBD] stage, column i), fully mature (Table 1, polar body 1 stage [PB1], column j), as well as total oocytes harvested (Table 1, column k), reported here as mean ± standard deviation for the number of stimulations performed (Table 1, column g). For each protocol, we also compared the average numbers of oocytes selected for ICSI fertilization (Table 1, column l) as well as the rates for producing fully expanded blastocysts following in vitro culture (Table 1, column m).
The first two stimulation protocols, used successfully with rhesus macaque females (Davenport et al., 2003; Hewitson et al., 2002; Hewitson et al., 1998; Nusser et al., 2001; Stouffer and Zelinski-Wooten, 2004), altered the inclusion of r-LH and/or GnRH agonist (Table 1). Both Protocol I and II gave equally high total oocyte yields at collection (29-33 total oocytes; Table 1, column k), but provided significantly lower yields of fully mature oocytes for fertilization compared to other stimulation protocols (average: 6-9 usable oocytes; 20-27% of the total oocytes collected; Table 1, column l; p= 0.05). We next investigated stimulation protocols that altered the r-LH administration without inclusion of the GnRH agonist and with increased r-hCG dosage to 2500 IU (Table 1; Protocols III, IV and V). For these, total oocyte yields increased overall (~35-49 oocytes; Table 1, column k), as did the average number of mature oocytes for fertilizations over Protocols I and II (average: 12-17 usable oocytes; 26-34% of the total collected; Table 1, column l; p=0.05). However, the best stimulation protocol for producing mature baboon oocytes for fertilization was Protocol VI, in which a full nine days of r-LH and GnRH agonist was used along with increasing r-hCG to 5000 IU (average total oocytes: ~68; average mature oocytes: ~29 usable oocytes; 43% of total oocytes collected; Table 1, column k; p= 0.05). Finally, since Baboons have slightly longer cycle lengths than macaques (33 days vs. 28 days; (Pope et al., 1982), we investigated if increasing the length of r-FSH/rLH stimulation beyond the typical 9 day regime was productive (Table 1; Protocols VII, VIII and VIX). We did not detect significant increases in total oocyte yields (average: 37-42 oocytes, Table 1, column k) or mature oocytes for fertilization (averages: 14-15 oocytes usable oocytes; 33-36% of total oocytes collected; Table 1, column l). Indeed, extending the hormonal stimulation protocol consistently produces poorer quality oocytes at collection than with other protocols (our unpublished data), although expanded blastocysts were produced (Table 1; column m).
Baboon stimulation protocols employed here show consistently lower yields in mature oocytes at metaphase II (MII) arrest compared to macaques (range in baboons: 20-43% MII (see also (Nyachieo et al., 2009c)); range in rhesus: 44-53% MII; (Hewitson et al., 1998; Nusser et al., 2001). Although r-FSH/r-LH produced several follicles per ovary >5mm on Day 11 of the stimulation protocol (average: 5-12; Dr. C. Castro, personnel communication), indicating good follicular recruitment, nearly two-thirds of the total harvested oocytes were immature GV stage oocytes. Increasing r-hCG up to 5000 IU (Table 1; column [e]), doses that was five times that reported for use in macaque stimulation protocols, only slightly improved the overall percentage of mature oocytes at aspiration. It is not currently understood why the r-hCG activity is inferior in the baboon. Most collected baboon GVs were fully grown, although attempts to mature these oocytes in vitro met with limited success (our unpublished observations).
Finally, the quality of oocytes harvested by the various stimulation protocols can be measured by their ability to produce expanded blastocysts following ICSI fertilization and development in vitro (Table 1, column m). Baboon blastocyst rates after 8-10 days of in vitro culture were consistently low (range: 0- 6%, Table 1, column m), regardless of the stimulation protocol employed, and much lower than those reported for rhesus macaques (40-60%; (Nusser et al., 2001)). The vast majority of the ICSI fertilized baboon oocytes were co-cultured on a customary NHP culture media, with buffalo rat liver cell monolayers in CMRL 1066 and fetal serum. Preliminary investigations on alternative media without co-culture, such as the human IVF sequential media G1/G2 or the single use media Global (LifeGlobal, ON, Canada), have shown only slight improvements in baboon in vitro developmental rates (our unpublished observations). Certainly, optimizing in vitro culture media will be paramount in improving preimplantation development in the baboon, as shown for other NHP species (Wolf, 2004). Interestingly baboon serum is superior to human serum or bovine serum albumin for inducing capacitation in baboon sperm (Nyachieo et al., 2009a; Nyachieo et al., 2009c).
Baboons are especially social animals and we investigated whether housing females in individual cages versus group caging (12-17 females + 1 vasectomized male) would impact ART outcomes (Table 2). We performed 49 stimulations of individually-caged females, collecting 2136 total oocytes (Table 2; columns b,c). Only about one-third (706) were fully mature at collection or within 6 hours post-aspiration (Table 2, column d). Nevertheless, an ICSI fertilization rate of 77% was observed (Table 2, column E), with 90% cleaving to the two cell stage (Table 2, column F). Subsequent in vitro development of fertilized zygotes derived from individually-caged females showed a significant decrease in cleavage rates (Table 2, columns G-K), with only 13 fully expanded blastocyst by Day 10 post-fertilization (2% of total fertilized 2PN zygotes; Table 2, column, L).
Table 2.
Comparison of Group Housed versus Single Housed Baboon Females on in Vitro Blastocyst and Embryonic Stem Cell Derivations.
| [a] Housing Type/Location | [b] # of Stim | [c] Total # Oocytes (avg #) | [d] Total Mature Oocytes for ICSI (% of total) | [e] # 2PN Fertilized (% of ICSI) | [f] # of 2-Cell Stage (% of Fert 2PN) | [g] # of 3-to-4 Cell Stage (% of Fert 2PN) | [h] # of 6-to-8 Cell Stage (% of Fert 2PN) | [i] # of 16-to- 32 Cell Stage (% of Fert 2PN) | [j] # of Morula Stage (% of Fert 2PN) | [k] # of Early Blastocyst (% of Fert 2PN) | [l] Expanded Blastocyst (% of Fert 2PN) | [m] ESC Lines (% of Exp. Blast.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Single/Indoor | 49 | 2136 | 706 (33) | 547 (77) | 494 (90) | 450 (82) | 385 (70) | 289 (53) | 180 (33) | 106 (19) | 13 (2) | 2 (15) |
| Group/Outdoor | 47 | 1746 | 587 (34) | 444 (76) | 405 (91) | 397 (89) | 335 (75) | 249 (56) | 182 (41) | 117 (26) | 9 (2) | 0 |
| Total | 96 | 3882 | 1293 (33) | 991 (77) | 899 (91) | 847 (85) | 720 (73) | 583 (54) | 362 (37) | 223 (23) | 22 (2) | 2 (9) |
For group housed females, we identified the cycle stage of individual females from the appearance of the sex skin tumescence. Menstruating females were administered hormonal shots using the chute system as described in the Methods section. From a total of 47 group housed females, we collected 1746 oocytes (Table 2, column b,c), slightly less than the total oocytes collected from single-housed females (45% versus 55%, respectively). The overall rates for mature oocytes collected, ICSI fertilization success, and first cleavage division did not differ significantly between group-housed and individually-caged females (Table 2, column d-f). Also, we observed similar declines in baboon in vitro development rates after second division (Table 2; columns g-l), with only 9 expanded blastocysts produced from normal ICSI fertilized oocytes in the group-housed females (2%; Table 2, column L).
In Vitro Fertilization and Preimplantation Development to Expanded Blastocysts
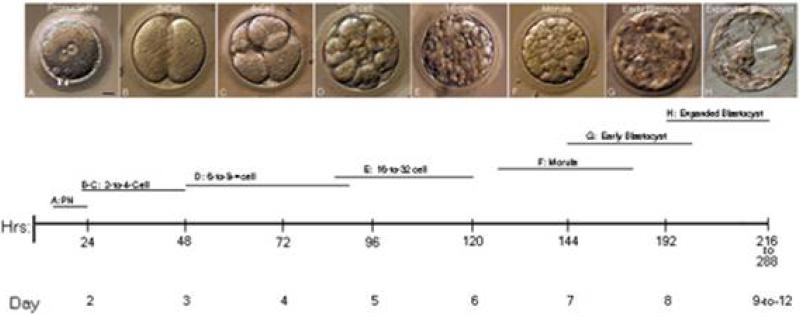
A timeline of baboon in vitro development is shown in Figure 1. The developmental characteristics of ICSI fertilized zygotes are similar to those reported for other macaques (Hewitson et al., 1998; Ng et al., 2002): male and female pronuclear formation occurs between 4-6 hrs post-ICSI fertilization, first cleavage between 22-48 hours post-ICSI, 8-cell stage by Day 4 post-fertilization, morula by Day 6-7 post-ICSI and expanded blastocyst by 8-12 days post-ICSI for embryos grown in the CMRL co-culture system. For derivation of baboon stem cell lines, day 9 expanded blastocysts are superior to earlier or later stage blastocysts (Simerly et al., 2009).
Figure 1.
Baboon Embryonic Development and Timeline after Intracytoplasmic Sperm Injection (ICSI). ICSI-derived baboon zygotes developed in vitro from the 1-cell zygote to the expanded blastocyst stage, the point where embryos can be used for immunosurgical isolation of the inner cell mass and attempted derivation of baboon embryonic stem cell lines. A: fertilized 2- pronuclear stage with two polar bodies (arrowheads); B: 2-cell; C: 4-cell; D: 8-cell; E: 16-cell; F: morula; G: early blastocyst with a small blastocoel; H: fully expanded blastocyst with prominent inner cell mass (arrow). Below: timeline in hours and days for various in vitro developmental stages in the baboon. The bars represent the developmental range for each stage observed from a minimum of six embryos. Bar in A: 20 μm.
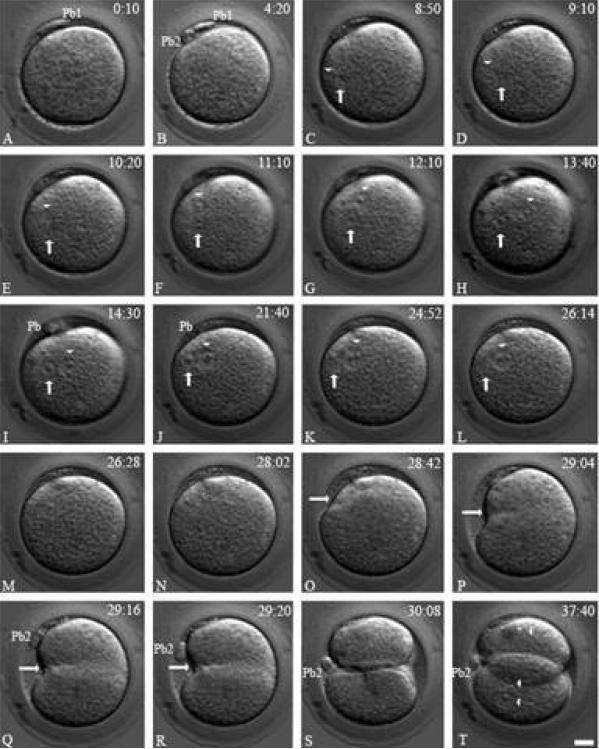
Analysis of the baboon ICSI fertilization process by TLVM is shown in Figure 2. Second polar body elicitation occurs between 2-4 hours post sperm injection (Fig. 2B, Pb2). The female pronucleus forms near the site of second polar body formation and rapidly migrates to the male sperm head, decondensing near the oocyte's cortex (Fig. 2C-D; arrowhead, female pronucleus; arrow, male pronucleus). Pronuclear apposition is nearly always eccentric in the cytoplasm and radial, with the male pronucleus nearest the cortical region and the female pronucleus distal (Fig. 2E-K). Pronuclear envelope breakdown (NEBD) is sequential but not simultaneous, with male NEBD generally preceding female NEBD by a few minutes (Fig. 2L-M, arrow: male pronucleus). Cleavage furrow initiation occurs predominately at the cortical region, adjacent to where the intact male pronucleus formerly resided in the cytoplasm prior to NEBD, and transverses the cytoplasm asymmetrically to the opposite cortex (Fig. 2N-R, arrow, cleavage furrow) as the polar bodies rotate into the furrow to lie between the interface of the opposing daughter nuclei (Fig. 2Q-S, Pb2). Nuclear reconstitution occurs in the daughter cells at the end of cell division, and occasional polyploidy blastomeres can be observed (Fig. 2T, arrowheads, nuclei). These early motility events accompanying the fertilization process are mirrored in macaque primates, but are distinct from other mammals, including humans (Simerly et al., 1998; Simerly et al., 1995; Simerly et al., 1999; Simerly et al., 1993).
Figure 2.
Time-lapse Video Microscopy of Baboon ICSI Fertilization. A: ICSI inseminated oocyte about 10 minutes post sperm injection. The first polar body is visible at the top (Pb1). B: within 4hr:20 min, the second polar body (Pb2) extrudes as the female pronucleus begins migration to the male (not shown). C: the apposed female (arrowhead) and male (arrow) pronuclei align eccentrically near the cortical region by 8hr: 50min within the activated cytoplasm. D-I : over the next 4.5 hrs, the tightly apposed male (arrows) and female pronuclei (arrowheads) begin to reorient within the activated egg's cytoplasm, rotating over 90° as pronuclear growth continues. J-K: as zygotic development continues, the apposed male and female pronuclei slightly shift towards the adjacent cortical region, with the male pronucleus (arrows) closest to the cortex and the female pronucleus (arrowheads) distal. Note the radial alignment of the pronuclei with the overlying cortex and nearly 90° alignment with the polar bodies (I-K; Pb). L-M: by 26hr:14min, nuclear envelope breakdown occurs in the male pronucleus (L: arrow) followed by female pronuclear envelop breakdown (M: arrowhead) 14 minutes later. N: mitotic zygote with no visible pronuclei at 28hr:02min. O-S: approximately 40 minutes later, an unequal cleavage furrow forms, beginning nearest the cortical region adjacent to the last position of the male pronucleus (arrows) and continuing to the opposite cortex. Note the second polar body (Pb2), tethered to the zygote, rotates into the cleavage furrow to lie between the daughter blastomeres (S: Pb2). T: daughter cells showing reformation of two nuclei in one blastomere (bottom: two arrowheads) and one nucleus in the other blastomere (upper: one arrowhead). Upper right: stage in hours: minutes post ICSI fertilization. Pb1: first polar body; Pb2: second polar body. Bar= 20 μm.
Embryo Transfer Results
To assess ART baboon embryo quality, growing embryos at various developmental stages were transferred to recipient females for pregnancy establishment (Table 3). Unlike other NHPs, baboon cycle staging for recipient can be done without invasive blood draws. Using careful daily recordings of the sex skin appearance over a period of months, a record of each female's predicted ovulation event can be determined with good accuracy (Hendrickx and Kraemer, 1968; Pope et al., 1982; Shaikh et al., 1982; Shearer et al., 1997; Zuckerman and Parkes, 1932). Also, in a majority of baboons, the cervix can be easily cannulated to perform noninvasive intrauterine embryo transfers. This latter advantage permits the transfer of late preimplantation stage embryos (i.e., morula and/or blastocyst) after in vitro culturing.
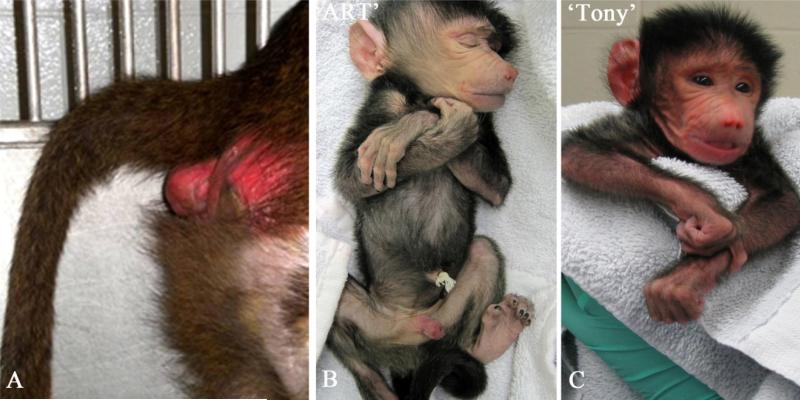
As shown in Table 3, twenty-six embryo transfers were performed on recipients of known fertility and who had previously successfully carried a fetus to term. Twenty-one laparoscopic transfers were performed using ICSI embryos between 1-cell-to-morula stages (Table 3, column 5). The recipient females ranged from 1-6 days post-ovulation, with the majority of cleavage stage embryos transferred into recipients at days 3-5 post-ovulation (Table 3, column 2). We observed 11/21 (52%) embryo transfers with suspected pregnancy call as visualized by skin coloration (Summary Table, column 4). A single pregnancy from 11 called pregnancies by sex skin coloration (9%) was successful carried to full term, produced by the transfer of six ICSI fertilized 1-cell zygotes into a Day 3 recipient (Table 3, column 7). As shown in Figure 3, this female recipient was called pregnant by skin coloration on Day 20 post-embryo transfers (Figure 3A) and twin male baboons that appeared morphologically normal were born 165 days post embryo transfers (Figure 3B-C; ‘Art’= 618 g; ‘Tony’=638 g).
Figure 3.
Sex Skin Coloration in Female Recipient 18437 at Day 30 post Embryo Transfer and Birth of Male Twins following ART. (A) The sex skin of baboon females provides a unique bioassay for pregnancy status (A: pink coloration indicating an on-going pregnancy) as well as stage in the menses cycle. This observational characteristic obviates the need to draw blood daily and then perform ELISA for hormone concentrations. (B-C): birth of twin male baboons from Recipient 18437 at 165 days post estimated gestational age. Images were taken within 3 hrs of birth. The twins were designated ‘ Art’ (B: for Assisted reproductive technologies) and ‘Tony’ (C: for San Antonio).
An additional 5 embryo transfers by intrauterine cannulation were performed on recipients 2-7 days post-ovulation (Table 3, columns 2 and 4) and with embryos from pre-morula through expanded blastocyst stage (Table 3, column 5). From 26 embryos transferred by intrauterine cannulation, a single recipient showed pregnancy sex skin coloration at Day 10 post transfer but this female spontaneously aborted on Day 12 (Table 3, Column 7). Pathology analysis of collected aborted tissue suggested the presence of both fetal and placental cells. Collectively, the results suggest that baboon ART, produces viable pregnancies leading to birth of normal young as well as the establishment of stable pluripotent stem cells lines (Simerly et al., 2009).
Discussion
The successful generation of live baboon offspring using all the methods involved in ART, including ICSI, is presented here. It is important to recognize that each nonhuman primate species requires optimization for ART protocols, and whereas success rates continue to improve with humans now thirty years after the introduction of ART, we may anticipate that additional research will improve upon the results presented here. Indeed, results with macaques started at modest levels and now as results have improved considerably, we have specialty rhesus which were generated by ART – including transgenic monkeys (Chan et al.), especially ones expressing Huntington's disease (Yang et al.), ones generated after cytoplasmic transplantation (Hewitson et al.), others using elongated spermatids and testicular sperm[ELSI; (Hewitson et al.)], some made after embryo splitting (Chan et al.), along with ones which discordant mitochondria (Tachibana et al.). Methods which generate live ART offspring in baboons, as shown here with twins born naturally, represent another step in the progression to develop and optimize protocols for utilization of this understudied primate for biomedical and behavioral research. It is certainly anticipated that the benefits of this model will encourage significant improvements in these procedures. These benefits which are complementary to rhesus, include the obvious changes in sex skin color as a bioassay in lieu of daily blood draws and ELIZA hormone testing; docile natures; straight cervix for non-surgical embryo transfer as well as eventually ultrasound-guided oocyte aspirations; ready availability of pedigreed colonies, cost-effectiveness, colonies expressing complex disease traits; evolutionary closeness to humans; and well-defined coat characteristics which differ among different strains.
Because of the history of discoveries with rhesus, sober arguments are necessary for justifying the development of a different – albeit related – Old World primate model. We propose that the NHP research community would prosper even more with the availabilities of reliable experimental protocols for baboons for several reasons that are all complementary to the rationales for rhesus and other macaque investigations. These include: the high cost of rhesus, which can be many times that of a similar baboon; the relative unavailability of rhesus compared with the readily available baboons; the advantages of the female baboons sex-skin, which serves as an obvious biomarker for menstrual cycle stage; the relative docility of baboons versus macaques; their relative similarities in size, anatomy and physiology to humans, as well as the availability of multiple generations of pedigreed baboons for which complex human disease phenotypes are being characterized. Protocols for baboon ART are now generating live offspring by ART and further improvements can be anticipated by this and other teams (e.g. Nyachieo et al., 2009a; (Nyachieo et al., 2009b). Yet, one reason why NHPs are not fully contributing to bridging the intellectual and biomedical gaps between rodents and people is because of continuing and significant limitations in NHP research resources. Our intent here has been to enrich the available research resources for reproductive, developmental, genetic and regenerative medical studies by adding baboons to our research armamentarium.
Baboons are even more closely related to humans than macaques (Rogers et al.; Stewart and Disotell). Yet macaques, especially rhesus monkeys of Indian origin, have earned the claim as the currently most popular NHP model for primate research. There are many justifications for this, including the decades of superb research that has established a strong foundation upon which other primate investigations can build their own research discoveries. These include areas of immunohistocompatibility (Dighe et al.; Rajesh et al.) as well as endometriosis (reviewed by (Banerjee and Fazleabas; D'Hooghe et al.). Though HIV/AIDS models in simians (Braun and Johnson, 2006) fall short of the perfect mark when matched with laboratory mice, in light of certain biomedical and preclinical fields, their utilities are inestimable. For example, the triplet nucleotide repeat diseases, including Huntington's, can be generated in mice but they do not display clinical phenotypes. Recently, a Huntington's rhesus model was generated that appeared to faithfully mirror the genetic and physiological ravages of this devastating neurodegenerative disease (Yang et al.). In stem cell research, investigators working with NHP have pioneered technologies later extrapolated to humans, including the first ESC derivations (Thomson and Marshall, 1998), parthenogenetic ESCs (Cibelli et al.) and even ESCs established from cloned blastocysts (Byrne et al., 2007).
Intrinsic interests in human biology, as well as important medical investigations, are generating increasing pressure to develop reliable and responsible fundamental and preclinical biological models. In this context, it may be worthwhile to briefly analyze the strengths of animals models that are currently popular and some of the reasons why they are now favored over their related predecessors. Mouse models, for which the Nobel Prizes in Physiology and Medicine were awarded in 2008, have unrivalled genetic pedigrees extending well over a century, unsurpassed transgenic and knock-out/in malleability, not to mention mouse ESCs unique capability to contribute to chimera with germ-line transmission. This, combined with their ease of care, cost effectiveness, public acceptance, and short generation time, have provided compelling justifications for their usage in place of the previously popular lab rat model. Similar genetic arguments, combined with cell and developmental advantages, supported the rationales for the zebrafish over other previously popular outbred fish, Xenopus spp over outbred Rana, as well as the nematode C. elegans over the previously outbred Ascaris parasite. (Boveri, 1901).
While the laboratory mouse will remain the mammal of first choice for biomedical studies, scientific gaps between them and humans mandate nonhuman primate models for several crucial fields, including reproductive and developmental biology (Hematti et al., 2005). Among the nonhuman primates, it is difficult indeed to envision an ape model for many ethical, practical and regulatory reasons. Lower primates, including lemurs, are of keen importance for evolutionary studies (Horvath and Willard, 2007). The New World Primates (NWP), including marmosets (Bankiewicz et al.), capuchins (Williams et al.), and squirrel monkeys (Abee, 2000), are a branch separate from the hominid lineage but continue to make important contributions. However, only Old World monkeys, which include macaques and baboons, are experimentally tractable species closely related in genetics, anatomy and physiology to humans (reviewed by (Wilmut and Taylor, 2007)).
Baboons now join macaques as Old World primate models for ART and other investigations in reproductive sciences, i.e. protocols for in vitro fertilization, intracytoplasmic sperm injection, preimplantation development to blastocysts, and birth of live young after embryo transfer are successful. This is important for several reasons, including the closer genetic proximity to humans of baboons compared to macaques like the well studied rhesus monkey. Furthermore, discoveries using discarded specimens from infertile patient-couples are leading to novel approaches for diagnosing idiopathic forms of human infertility (Tachibana et al., 2009b), and primate confirmations are important. Finally, the availability of the baboon as a research resource complements the more limited macaque.
Acknowledgments
We gratefully acknowledge the leadership and research support of colleagues at the Southwest NPRC, including Drs. John VandeBerg, Tom Folks, William Cummins, and Karen Rice; and John Tobler, Felicia Aguillon, Kyle Ponce, Michael Washington, Annessa Raiford, Wade Hodgson, Terry Naegelin, and Sharon Price for project coordination and animal care support. We also thank Dr. Cecelia Penedo at the California NPRC for genetic confirmation of the baboon twin's pedigrees, Dr. Diane Carlisle for helpful suggestions with manuscript preparation and Ms. Angela Palermo for administrative support; and Dr. Roger Pedersen at Cambridge University, Dr. Peter Donovan at University of California-Irvine and Stacie Oliver at the Pittsburgh Development Center for manuscript review.
Grant Support: We gratefully acknowledge grant support from the NIH: P01 HD47675; R37 HD12913; R24 RR13632 to Gerald Schatten, as well as NIH sponsorship for the Southwest National Primate Research Center.
References
- Abee CR. Squirrel monkey (Saimiri spp.) research and resources. ILAR J. 2000;41:2–9. doi: 10.1093/ilar.41.1.2. [DOI] [PubMed] [Google Scholar]
- Ahrens ET, Srinivas M, Capuano S, Simhan HN, Schatten GP. Magnetic resonance imaging of embryonic and fetal development in model systems. Methods Mol Med. 2006;124:87–101. doi: 10.1385/1-59745-010-3:87. [DOI] [PubMed] [Google Scholar]
- Banaszak S, Brudney A, Donnelly K, Chai D, Chwalisz K, Fazleabas AT. Modulation of the action of chorionic gonadotropin in the baboon (Papio anubis) uterus by a progesterone receptor antagonist (ZK 137. 316). Biol Reprod. 2000;63:820–825. doi: 10.1095/biolreprod63.3.820. [DOI] [PubMed] [Google Scholar]
- Banerjee P, Fazleabas AT. Endometrial responses to embryonic signals in the primate. Int J Dev Biol. 2009;54:295–302. doi: 10.1387/ijdb.082829pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankiewicz KS, Sanchez-Pernaute R, Oiwa Y, Kohutnicka M, Cummins A, Eberling J. Preclinical models of Parkinson's disease. Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0904s09. Chapter 9, Unit 4. [DOI] [PubMed] [Google Scholar]
- Basatemur E, Sutcliffe A. Follow-up of children born after ART. Placenta. 2008;29(Suppl B):135–140. doi: 10.1016/j.placenta.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Bavister BD. How animal embryo research led to the first documented human IVF. Reprod Biomed Online. 2002;4(Suppl 1):24–29. doi: 10.1016/s1472-6483(12)60008-x. [DOI] [PubMed] [Google Scholar]
- Bavister BD, Boatman DE, Leibfried L, Loose M, Vernon MW. Fertilization and cleavage of rhesus monkey oocytes in vitro. Biology of reproduction. 1983;28:983. doi: 10.1095/biolreprod28.4.983. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Parfitt DE, Zernicka-Goetz M. Formation of the embryonic-abembryonic axis of the mouse blastocyst: relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Development. 2008;135:953–962. doi: 10.1242/dev.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveri T. Zellen-studien: Uber die Natur der Centrosomen. IV. Jena; Germany: 1901. [Google Scholar]
- Bowles EJ, Tecirlioglu RT, French AJ, Holland MK, St John JC. Mitochondrial DNA transmission and transcription after somatic cell fusion to one or more cytoplasts. Stem Cells. 2008;26:775–782. doi: 10.1634/stemcells.2007-0747. [DOI] [PubMed] [Google Scholar]
- Braun SE, Johnson RP. Setting the stage for bench-to-bedside movement of anti-HIV RNA inhibitors-gene therapy for AIDS in macaques. Front Biosci. 2006;11:838–851. doi: 10.2741/1841. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- Chai D, Cuneo S, Falconer H, Mwenda JM, D'Hooghe T. Olive baboon (Papio anubis anubis) as a model for intrauterine research. J Med Primatol. 2007;36:365–369. doi: 10.1111/j.1600-0684.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- Chan AW, Dominko T, Luetjens CM, Neuber E, Martinovich C, Hewitson L, Simerly CR, Schatten GP. Clonal propagation of primate offspring by embryo splitting. Science. 2000;287:317–319. doi: 10.1126/science.287.5451.317. [DOI] [PubMed] [Google Scholar]
- Cibelli JB, Grant KA, Chapman KB, Cunniff K, Worst T, Green HL, Walker SJ, Gutin PH, Vilner L, Tabar V, et al. Parthenogenetic stem cells in nonhuman primates. Science. 2002;295:819. doi: 10.1126/science.1065637. [DOI] [PubMed] [Google Scholar]
- Cox LA, Birnbaum S, Mahaney MC, Rainwater DL, Williams JT, VandeBerg JL. Identification of promoter variants in baboon endothelial lipase that regulate high-density lipoprotein cholesterol levels. Circulation. 2007;116:1185–1195. doi: 10.1161/CIRCULATIONAHA.107.704346. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am J Obstet Gynecol. 1995;173:125–134. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Debrock S, Kyama CM, Chai DC, Cuneo S, Hill JA, Mwenda JM. Baboon model for fundamental and preclinical research in endometriosis. Gynecol Obstet Invest. 2004a;57:43–46. [PubMed] [Google Scholar]
- D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–161. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Spiessens C, Chai DC, Mwethera PG, Makokha AO, Mwenda JM. Ovarian stimulation, egg aspiration, in vitro fertilization and embryo transfer in the baboon (Papio anubis): a pilot project at the Institute of Primate Research, Nairobi, Kenya. Gynecol Obstet Invest. 2004b;57:23–26. [PubMed] [Google Scholar]
- Davenport AT, Lees CJ, Green HL, Grant KA. Long-acting depot formulation of luprolide acetate as a method of hypothalamic down regulation for controlled ovarian hyperstimulation and oocyte production in Macaca fascicularis. Biol Reprod. 2003;68:2261–2266. doi: 10.1095/biolreprod.102.012468. [DOI] [PubMed] [Google Scholar]
- Dighe V, Clepper L, Pedersen D, Byrne J, Ferguson B, Gokhale S, Penedo MC, Wolf D, Mitalipov S. Heterozygous embryonic stem cell lines derived from nonhuman primate parthenotes. Stem Cells. 2008;26:756–766. doi: 10.1634/stemcells.2007-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RG, Steptoe PC, Purdy JM. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980;87:737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Elster N. Less is more: the risks of multiple births. The Institute for Science, Law, and Technology Working Group on Reproductive Technology. Fertil Steril. 2000;74:617–623. doi: 10.1016/s0015-0282(00)00713-5. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Physiology and pathology of implantation in the human and nonhuman primate. Semin Reprod Med. 2007;25:405–409. doi: 10.1055/s-2007-991037. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Strakova Z. Endometrial function: cell specific changes in the uterine environment. Mol Cell Endocrinol. 2002;186:143–147. doi: 10.1016/s0303-7207(01)00655-4. [DOI] [PubMed] [Google Scholar]
- French AJ, Adams CA, Anderson LS, Kitchen JR, Hughes MR, Wood SH. Development of human cloned blastocysts following somatic cell nuclear transfer with adult fibroblasts. Stem Cells. 2008;26:485–493. doi: 10.1634/stemcells.2007-0252. [DOI] [PubMed] [Google Scholar]
- Gardner RL. The axis of polarity of the mouse blastocyst is specified before blastulation and independently of the zona pellucida. Hum Reprod. 2007;22:798–806. doi: 10.1093/humrep/del424. [DOI] [PubMed] [Google Scholar]
- Gerris J, Van Royen E. Avoiding multiple pregnancies in ART: a plea for single embryo transfer. Hum Reprod. 2000;15:1884–1888. doi: 10.1093/humrep/15.9.1884. [DOI] [PubMed] [Google Scholar]
- Havill LM, Mahaney MC, Cox LA, Morin PA, Joslyn G, Rogers J. A quantitative trait locus for normal variation in forearm bone mineral density in pedigreed baboons maps to the ortholog of human chromosome 11q. J Clin Endocrinol Metab. 2005;90:3638–3645. doi: 10.1210/jc.2004-1618. [DOI] [PubMed] [Google Scholar]
- Havill LM, Mahaney MC, Czerwinski SA, Carey KD, Rice K, Rogers J. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33:877–878. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- Hematti P, Obrtlikova P, Kaufman DS. Nonhuman primate embryonic stem cells as a preclinical model for hematopoietic and vascular repair. Exp Hematol. 2005;33:980–986. doi: 10.1016/j.exphem.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Hendrickx AG, Kraemer DC. Preimplantation stages of baboon embryos (Papio sp.). Anat Rec. 1968;162:111–120. doi: 10.1002/ar.1091620110. [DOI] [PubMed] [Google Scholar]
- Hewitson L. Primate models for assisted reproductive technologies. Reproduction. 2004;128:293–299. doi: 10.1530/rep.1.00242. [DOI] [PubMed] [Google Scholar]
- Hewitson L, Dominko T, Takahashi D, Martinovich C, Ramalho-Santos J, Sutovsky P, Fanton J, Jacob D, Monteith D, Neuringer M, et al. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat Med. 1999;5:431–433. doi: 10.1038/7430. [DOI] [PubMed] [Google Scholar]
- Hewitson L, Martinovich C, Simerly C, Takahashi D, Schatten G. Rhesus offspring produced by intracytoplasmic injection of testicular sperm and elongated spermatids. Fertil Steril. 2002;77:794–801. doi: 10.1016/s0015-0282(01)03281-2. [DOI] [PubMed] [Google Scholar]
- Hewitson L, Simerly C, Dominko T, Schatten G. Cellular and molecular events after in vitro fertilization and intracytoplasmic sperm injection. Theriogenology. 2000;53:95–104. doi: 10.1016/s0093-691x(99)00243-5. [DOI] [PubMed] [Google Scholar]
- Hewitson L, Takahashi D, Dominko T, Simerly C, Schatten G. Fertilization and embryo development to blastocysts after intracytoplasmic sperm injection in the rhesus monkey. Hum Reprod. 1998;13:3449–3455. doi: 10.1093/humrep/13.12.3449. [DOI] [PubMed] [Google Scholar]
- Hlusko LJ, Do N, Mahaney MC. Genetic correlations between mandibular molar cusp areas in baboons. Am J Phys Anthropol. 2007;132:445–454. doi: 10.1002/ajpa.20528. [DOI] [PubMed] [Google Scholar]
- Horvath JE, Willard HF. Primate comparative genomics: lemur biology and evolution. Trends Genet. 2007;23:173–182. doi: 10.1016/j.tig.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Iannaccone PM, Jacob HJ. Rats! Dis Model Mech. 2009;2:206–210. doi: 10.1242/dmm.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder T, Neil J, Yoder B, Rees S. Non-human primate models of neonatal brain injury. Semin Perinatol. 2004;28:396–404. doi: 10.1053/j.semperi.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Khalaf Y, El-Toukhy T, Coomarasamy A, Kamal A, Bolton V, Braude P. Selective single blastocyst transfer reduces the multiple pregnancy rate and increases pregnancy rates: a pre- and postintervention study. BJOG. 2008;115:385–390. doi: 10.1111/j.1471-0528.2007.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili MA, Aflatoonian A, Zavos PM. Intracytoplasmic injection using spermatids and subsequent pregnancies: round versus elongated spermatids. J Assist Reprod Genet. 2002;19:84–86. doi: 10.1023/A:1014447731630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Yanagimachi R. Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biol Reprod. 1995;53:855–862. doi: 10.1095/biolreprod53.4.855. [DOI] [PubMed] [Google Scholar]
- Kloc M, Jaglarz M, Dougherty M, Stewart MD, Nel-Themaat L, Bilinski S. Mouse early oocytes are transiently polar: three-dimensional and ultrastructural analysis. Exp Cell Res. 2008;314:3245–3254. doi: 10.1016/j.yexcr.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Sato A, Otsu E, Hiura H, Tomatsu C, Utsunomiya T, Sasaki H, Yaegashi N, Arima T. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum Mol Genet. 2007;16:2542–2551. doi: 10.1093/hmg/ddm187. [DOI] [PubMed] [Google Scholar]
- Li C, Yang Y, Gu J, Ma Y, Jin Y. Derivation and transcriptional profiling analysis of pluripotent stem cell lines from rat blastocysts. Cell Res. 2009;19:173–186. doi: 10.1038/cr.2008.301. [DOI] [PubMed] [Google Scholar]
- Li S, Li Y, Du W, Zhang L, Yu S, Dai Y, Zhao C, Li N. Aberrant gene expression in organs of bovine clones that die within two days after birth. Biol Reprod. 2005;72:258–265. doi: 10.1095/biolreprod.104.029462. [DOI] [PubMed] [Google Scholar]
- Lim D, Bowdin SC, Tee L, Kirby GA, Blair E, Fryer A, Lam W, Oley C, Cole T, Brueton LA, et al. Clinical and molecular genetic features of Beckwith-Wiedemann syndrome associated with assisted reproductive technologies. Hum Reprod. 2008 doi: 10.1093/humrep/den406. [DOI] [PubMed] [Google Scholar]
- Lin G, OuYang Q, Zhou X, Gu Y, Yuan D, Li W, Liu G, Liu T, Lu G. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Res. 2007;17:999–1007. doi: 10.1038/cr.2007.97. [DOI] [PubMed] [Google Scholar]
- Mai Q, Yu Y, Li T, Wang L, Chen MJ, Huang SZ, Zhou C, Zhou Q. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell Res. 2007;17:1008–1019. doi: 10.1038/cr.2007.102. [DOI] [PubMed] [Google Scholar]
- Motosugi N, Dietrich JE, Polanski Z, Solter D, Hiiragi T. Space asymmetry directs preferential sperm entry in the absence of polarity in the mouse oocyte. PLoS Biol. 2006;4:e135. doi: 10.1371/journal.pbio.0040135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SC, Martelli P, Liow SL, Herbert S, Oh SH. Intracytoplasmic injection of frozen-thawed epididymal spermatozoa in a nonhuman primate model, the cynomolgus monkey (Macaca fascicularis). Theriogenology. 2002;58:1385–1397. doi: 10.1016/s0093-691x(02)01035-x. [DOI] [PubMed] [Google Scholar]
- Nusser KD, Mitalipov S, Widmann A, Gerami-Naini B, Yeoman RR, Wolf DP. Developmental competence of oocytes after ICSI in the rhesus monkey. Hum Reprod. 2001;16:130–137. doi: 10.1093/humrep/16.1.130. [DOI] [PubMed] [Google Scholar]
- Nyachieo A, Spiessens C, Chai DC, Kiulia NM, Mwenda JM, D'Hooghe TM. Baboon serum is superior to human or bovine serum albumin for baboon sperm capacitation and zona binding. J Med Primatol. 2009a;38:145–150. doi: 10.1111/j.1600-0684.2008.00319.x. [DOI] [PubMed] [Google Scholar]
- Nyachieo A, Spiessens C, Chai DC, Mwenda JM, D'Hooghe TM. Menstrual cycle synchronization, ovarian stimulation, and in vitro fertilization in olive baboons (Papio anubis): a prospective randomized study. Fertil Steril. 2009b;91:602–610. doi: 10.1016/j.fertnstert.2007.11.071. [DOI] [PubMed] [Google Scholar]
- Nyachieo A, Spiessens C, Mwenda JM, Debrock S, D'Hooghe TM. Improving ovarian stimulation protocols for IVF in baboons: lessons from humans and rhesus monkeys. Anim Reprod Sci. 2009c;110:187–206. doi: 10.1016/j.anireprosci.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Ogura A, Matsuda J, Yanagimachi R. Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc Natl Acad Sci U S A. 1994;91:7460–7462. doi: 10.1073/pnas.91.16.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka-Goetz M. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci. 2005a;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- Plusa B, Hadjantonakis AK, Gray D, Piotrowska-Nitsche K, Jedrusik A, Papaioannou VE, Glover DM, Zernicka-Goetz M. The first cleavage of the mouse zygote predicts the blastocyst axis. Nature. 2005b;434:391–395. doi: 10.1038/nature03388. [DOI] [PubMed] [Google Scholar]
- Pope CE, Pope VZ, Beck LR. Development of baboon preimplantation embryos to post-implantation stages in vitro. Biol Reprod. 1982;27:915–923. doi: 10.1095/biolreprod27.4.915. [DOI] [PubMed] [Google Scholar]
- Rajesh D, Chinnasamy N, Mitalipov SM, Wolf DP, Slukvin I, Thomson JA, Shaaban AF. Differential requirements for hematopoietic commitment between human and rhesus embryonic stem cells. Stem Cells. 2007;25:490–499. doi: 10.1634/stemcells.2006-0277. [DOI] [PubMed] [Google Scholar]
- Redl H, Bahrami S. Large animal models: baboons for trauma, shock, and sepsis studies. Shock. 2005;24(Suppl 1):88–93. doi: 10.1097/01.shk.0000191339.46777.63. [DOI] [PubMed] [Google Scholar]
- Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, Pryzhkova MV, Revazova ES, Turovets NA, Kochetkova OD, et al. Patient-specific stem cell lines derived from human parthenogenetic blastocysts: HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–449. doi: 10.1089/clo.2007.0033. [DOI] [PubMed] [Google Scholar]
- Rietzler M, Bittner M, Kolanus W, Schuster A, Holzmann B. The human WD repeat protein WAIT-1 specifically interacts with the cytoplasmic tails of beta7-integrins. J Biol Chem. 1998;273:27459–27466. doi: 10.1074/jbc.273.42.27459. [DOI] [PubMed] [Google Scholar]
- Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- Rogers J, Hixson JE. Baboons as an animal model for genetic studies of common human disease. Am J Hum Genet. 1997;61:489–493. doi: 10.1086/515527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Mahaney MC, Witte SM, Nair S, Newman D, Wedel S, Rodriguez LA, Rice KS, Slifer SH, Perelygin A, et al. A genetic linkage map of the baboon (Papio hamadryas) genome based on human microsatellite polymorphisms. Genomics. 2000;67:237–247. doi: 10.1006/geno.2000.6245. [DOI] [PubMed] [Google Scholar]
- Shaikh A, Shaikh S, Celaya C, Goldzieher J. Ovulation Pattern in Successive Cycles in the Baboon. Primates. 1982;23:592–959. [Google Scholar]
- Shearer MH, Lucas AH, Anderson PW, Carey KD, Jenson HB, Chanh TC, Stanley JR, Kennedy RC. The baboon as a nonhuman primate model for assessing the effects of maternal immunization with Haemophilus influenzae type b polysaccharide vaccines. Infection and Immunity. 1997;65:3267–3270. doi: 10.1128/iai.65.8.3267-3270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C, Nowak G, de Lanerolle P, Schatten G. Differential expression and functions of cortical myosin IIA and IIB isotypes during meiotic maturation, fertilization, and mitosis in mouse oocytes and embryos. Mol Biol Cell. 1998;9:2509–2525. doi: 10.1091/mbc.9.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly C, Wu GJ, Zoran S, Ord T, Rawlins R, Jones J, Navara C, Gerrity M, Rinehart J, Binor Z. The paternal inheritance of the centrosome, the cell's microtubule-organizing center, in humans, and the implications for infertility. Nat Med. 1995;1:47–52. doi: 10.1038/nm0195-47. [DOI] [PubMed] [Google Scholar]
- Simerly C, Zoran SS, Payne C, Dominko T, Sutovsky P, Navara CS, Salisbury JL, Schatten G. Biparental inheritance of gamma-tubulin during human fertilization: molecular reconstitution of functional zygotic centrosomes in inseminated human oocytes and in cell-free extracts nucleated by human sperm. Mol Biol Cell. 1999;10:2955–2969. doi: 10.1091/mbc.10.9.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly CR, Hecht NB, Goldberg E, Schatten G. Tracing the incorporation of the sperm tail in the mouse zygote and early embryo using an anti-testicular alpha-tubulin antibody. Dev Biol. 1993;158:536–548. doi: 10.1006/dbio.1993.1211. [DOI] [PubMed] [Google Scholar]
- Simerly CR, Navara CS, Castro CA, Turpin JC, Redinger CJ, Mich-Basso JD, Jacoby ES, Grund KJ, McFarland DA, Oliver SL, et al. Establishment and characterization of baboon embryonic stem cell lines: An Old World Primate model for regeneration and transplantation research. Stem Cell Res. 2009;2:178–187. doi: 10.1016/j.scr.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John JC, Moffatt O, D'Souza N. Aberrant heteroplasmic transmission of mtDNA in cloned pigs arising from double nuclear transfer. Mol Reprod Dev. 2005;72:450–460. doi: 10.1002/mrd.20370. [DOI] [PubMed] [Google Scholar]
- Steptoe P, Edwards R. Pregnancy in an infertile patient after transfer of an embryo fertilised in vitro. Br Med J (Clin Res Ed) 1983;286:1351–1352. doi: 10.1136/bmj.286.6374.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG, Walters DE. Observations on 767 clinical pregnancies and 500 births after human in-vitro fertilization. Hum Reprod. 1986;1:89–94. doi: 10.1093/oxfordjournals.humrep.a136366. [DOI] [PubMed] [Google Scholar]
- Stevens VC. Some reproductive studies in the baboon. Hum Reprod Update. 1997;3:533–540. doi: 10.1093/humupd/3.6.533. [DOI] [PubMed] [Google Scholar]
- Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998;8:R582–588. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Stojkovic P, Leary C, Hall VJ, Armstrong L, Herbert M, Nesbitt M, Lako M, Murdoch A. Derivation of a human blastocyst after heterologous nuclear transfer to donated oocytes. Reprod Biomed Online. 2005;11:226–231. doi: 10.1016/s1472-6483(10)60962-5. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol. 2004;2:32. doi: 10.1186/1477-7827-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009a doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Terada Y, Ogonuki N, Ugajin T, Ogura A, Murakami T, Yaegashi N, Okamura K. Functional assessment of centrosomes of spermatozoa and spermatids microinjected into rabbit oocytes. Mol Reprod Dev. 2009b;76:270–277. doi: 10.1002/mrd.20951. [DOI] [PubMed] [Google Scholar]
- Tejero ME, Voruganti VS, Rodriguez-Sanchez IP, Proffitt JM, Blangero J, Cox LA, Mahaney MC, Rogers J, VandeBerg JL, Cole SA, et al. Genetics of variation in adiponectin in pedigreed baboons: evidence for pleiotropic effects on adipocyte volume and serum adiponectin. Heredity. 2008;100:382–389. doi: 10.1038/sj.hdy.6801089. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Marshall VS. Primate embryonic stem cells. Current topics in developmental biology. 1998;38:133–165. doi: 10.1016/s0070-2153(08)60246-x. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Sperm centrosome dysfunction: a possible new class of male factor infertility in the human. Mol Hum Reprod. 1996;2:349–354. doi: 10.1093/molehr/2.5.349. [DOI] [PubMed] [Google Scholar]
- VandeBerg JF, Williams-Blangero S, Tardif SD. The Baboon in Biomedical Research Series: Developments in Primatology: Progress and Prospects. Springer; New York, New York: 2008. [Google Scholar]
- Vinson A, Mahaney MC, Cox LA, Rogers J, VandeBerg JL, Rainwater DL. A pleiotropic QTL on 2p influences serum Lp-PLA2 activity and LDL cholesterol concentration in a baboon model for the genetics of atherosclerosis risk factors. Atherosclerosis. 2008;196:667–673. doi: 10.1016/j.atherosclerosis.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Baptista PM, Daunais JB, Szeliga KT, Friedman DP, Soker S. The effects of ethanol consumption on vasculogenesis potential in nonhuman primates. Alcohol Clin Exp Res. 2008;32:155–161. doi: 10.1111/j.1530-0277.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Taylor J. Stem cells: primates join the club. Nature. 2007;450:485–486. doi: 10.1038/450485a. [DOI] [PubMed] [Google Scholar]
- Wolf DP. Assisted reproductive technologies in rhesus macaques. Reprod Biol Endocrinol. 2004;2:37. doi: 10.1186/1477-7827-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Cheng PH, Banta H, Piotrowska-Nitsche K, Yang JJ, Cheng EC, Snyder B, Larkin K, Liu J, Orkin J, et al. Towards a transgenic model of Huntington's disease in a non-human primate. Nature. 2008;453:921–924. doi: 10.1038/nature06975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Raabe T, Hecht NB. MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol Reprod. 2005;73:427–433. doi: 10.1095/biolreprod.105.040998. [DOI] [PubMed] [Google Scholar]
- Zuckerman S, Parkes A. The social life of monkeys and apes. Proc Zool Soc. Trubner and Company; London, Kegan Paul, Trench: 1932. p. XII.p. 357. [Google Scholar]