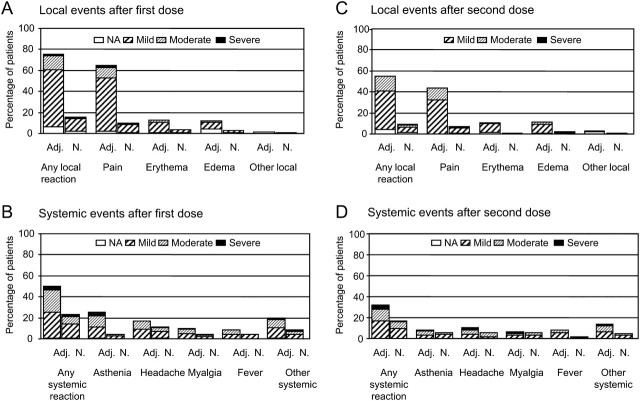
Figure 2.
Solicited reports of adverse events 21 days after the first dose and second dose of the H1N1 vaccine. Patients with at least 1 vaccine-related adverse event are reported. Severity of vaccine-related adverse events was graded as mild (mild or transient discomfort, without limitation of normal daily activities, and no medical intervention or corrective treatment required), moderate (mild to moderate limitation of normal daily activities and minimal medical intervention or corrective treatment required), or severe (marked limitation of normal daily activities, medical intervention and corrective treatment required, and possible hospitalization), except for erythema and edema at the injection site, which was graded on the basis of a measurement of the diameter of the local reaction (mild, 0–50 mm; moderate, 50–100 mm; severe, >100 mm). *Other severe systemic reactions in the adjuvanted vaccine group after receipt of the first dose: oral herpes, rhinorrhea, and arthralgia, with 1 patient who reported multiple events (arthralgia, shivering, rhinorrhea, back pain, abdominal pain, cough, dyspnea, nasal congestion, and pharyngolaryngeal pain; day 1 to day 10); other severe systemic reactions in the nonadjuvanted vaccine group: arthralgia, cough, and hot flash. †Other severe systemic reactions in the adjuvanted vaccine group after receipt of the second dose: arthralgia in 2 patients, shivering; other severe systemic reactions in the nonadjuvanted vaccine group: shivering. Adj, AS03A-adjuvanted vaccine group; N, nonadjuvanted-vaccine group; NA, not available.