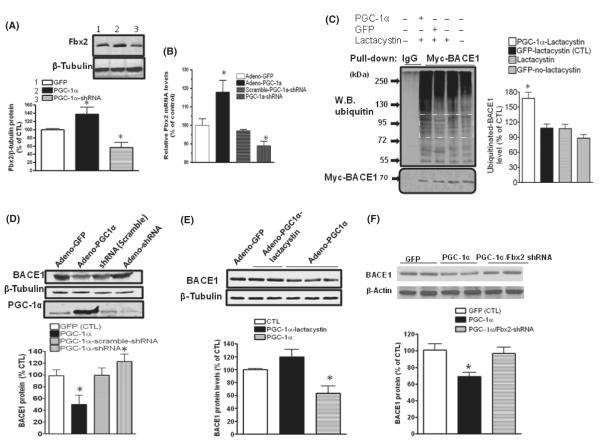
Fig. 4.
PGC-1α-mediated BACE1 degradation through Fbx2. Hippocampal neurons derived from Tg2576 embryos were infected with adenoviral GFP, adenoviral PGC-1α, PGC-1α shRNA or scrambled shRNA, respectively. (A) Forty-eight hours after infection, Fbx2 protein levels were probed using anti-Fbx2 antibody. PGC-1α markedly increases Fbx2 protein expression (lane 2), while adenoviral-PGC-1α shRNA reduces the Fbx2 levels (lane 3) (N = 5, means ± SEM, *P < 0.05. (B) Total RNA was extracted from transfected cells. Levels of Fbx2 mRNA were quantified by qRT-PCR (n = 5 independent studies; *P < 0.05). (C) PGC-1α promotes BACE1 ubiquitination. HEK293 cells stably expressing Myc-BACE1 were infected with adenoviral-PGC-1α or adenoviral-GFP constructs and treated with 5 μm lactacystin. BACE1 was immunoprecipitated with an antibody against Myc, The ubiquitinated BACE1 was probed with anti-ubiquitin antibody. The levels of ubiquitinated BACE1 levels in the square area were quantified and represented in the graph (right panel). Data are means ± SEM of the results from two independent experiments; *P < 0.05 compared with adenoviral-GFP/lactacystin-treated cells (control). (D) Silencing endogenous PGC-1α blocks BACE1 degradation. Neurons were infected with adenoviral GFP, adenoviral PGC-1α, and adenoviral PGC-1α shRNA; (E) lactacystin blocked the effects of PGC-1α on BACE1 degradation. (F) Silencing Fbx2 diminishes the effects of PGC-1α on BACE1 degradation. (n = 5, data are means ± SEM; n = 5 per culture; *P < 0.05 relative to adenoviral-GFP-infected cell cultures).