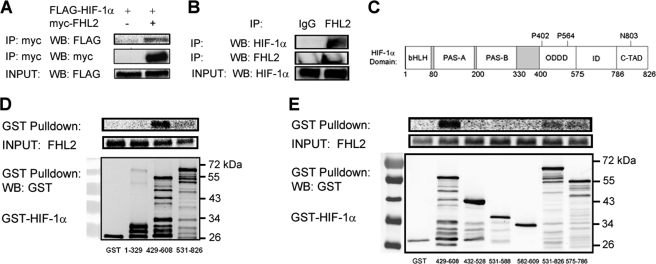
FIGURE 1.
FHL2 interacts with HIF-1α. A, FLAG-HIF-1α co-immunoprecipitates with FHL2. 293T cells were co-transfected with expression vectors encoding FLAG-HIF-1α and either EV or vector encoding Myc epitope-tagged FHL2. At 24 h post-transfection, the cells were lysed and the lysates were immunoprecipitated with anti-Myc antibody. IP products and cell lysates were subjected to WB with anti-Myc or anti-FLAG antibody. B, endogenous FHL2 co-immunoprecipitates with endogenous HIF-1α. Hep3B cells were exposed to 1% O2 for 6 h, lysed, and subjected to immunoprecipitation with anti-IgG or anti-FHL2 antibody. IP products were subjected to WB with anti-FHL2 or anti-HIF-1α antibody. C, the location of the basic helix-loop-helix domain (bHLH), Per-Arnt-Sim homology domain (PAS), O2-dependent degradation domain (ODDD), inhibitory domain (ID), and C-TAD of HIF-1α are shown. Sites of prolyl (P) and asparaginyl (N) hydroxylation are indicated. D and E, FHL2 interacts specifically and directly with HIF-1α(429–608). Purified GST and GST fusion proteins containing the indicated amino acid residues of HIF-1α were incubated with in vitro transcribed, in vitro translated, and 35S-labeled FHL2, captured with glutathione-Sepharose beads and analyzed by SDS-PAGE and autoradiography (top panels) or by WB with GST antibody (bottom panel).