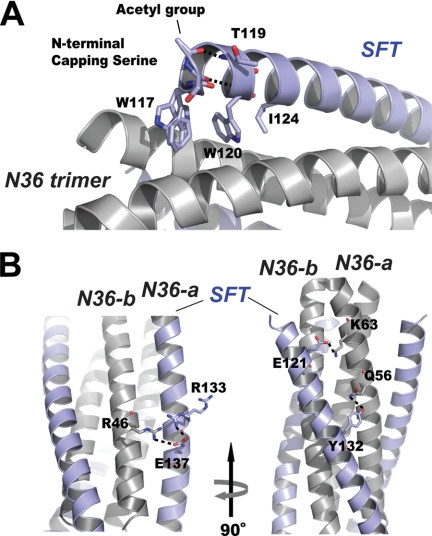
FIGURE 3.
Interactions between SFT and N36 peptides. A, portion of the ribbon model of 6-HB formed by SFT/N36 with the labels. Residues at the N terminus of SFT are shown in stick model (colored by elements). Trp-117, Trp-120, and Ile-124 were unchanged in SFT design, forming the typical pocket binding domain. The side chain of Thr-119 is located above the pocket binding domain, serving to stabilize the hydrophobic pocket. The carbonyl group of the capping acetylserine at the N terminus of SFT accepts a hydrogen bond (dashed line) from the NH group of Trp-120; the N-terminal acetyl group of the capping serine accepts another hydrogen bond (dashed line) from the NH group of Thr-119. B, portion of ribbon model of the 6-HB formed by SFT/N36 with the labels. Model on the right side is the different view of the model on the left side (rotation 90° around vertical axis to the left). Residues involving salt bridging and hydrogen bonding between SFT and two adjacent N36-a N36-b, are shown as a stick model. The salt bridges and hydrogen bond are indicated with dashed lines.