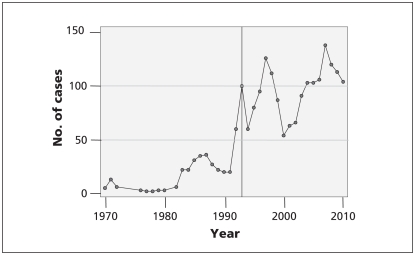
Figure 1:
Number of federal court cases in which a generic drug firm was either an applicant or a respondent, 1970–2010. The line represents the introduction of Notice of Complience regulations in 1993. Source: court index and docket of federal court and federal court of appeal (http://cas-ncr-nter03.cas-satj.gc.ca/IndexingQueries/infp_queries_e.php).