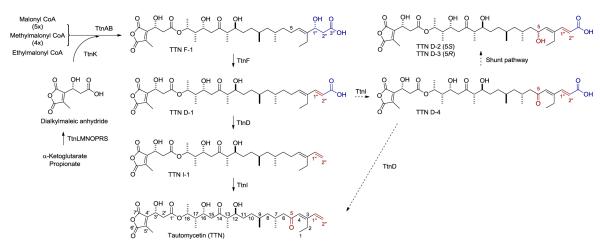
Figure 1.
Proposed biosynthetic pathway for TTN in S. griseochromogenes featuring (i) TtnKLMNOPRS-catalyzed biosynthesis of the dialkylmaleic anhydride moiety from one molecule each of α-ketoglutarate and propionate and its subsequent incorporation into the TTN polyketide scaffold, (ii) TtnAB-catalyzed assembly of the TTN polyketide scaffold from five malonyl CoAs, four methylmalonyl CoAs, and one ethylmalonyl CoA, and (iii) TtnFDI-catalyzed tailoring steps of TTN F-1 to TTN in the order of C-1”/C-2” dehydration by TtnF, C-3” decarboxylation by TtnD, and C-5 oxidation by TtnI. Solid arrows, main pathway; dashed line arrows, side steps due to substrate promiscuity of TtnD and TtnI. TTN D-2 and TTN D-3 are shunt metabolites most likely resulted from reduction of TTN D-4 by adventitious activities in S. griseochromogenes fermentation.