Abstract
A direct correlation exists between the level of histone H4 hyperacetylation induced by sodium butyrate and the extent to which nucleosomes lose their compact shape and become elongated (62.0% of the particles have a length/width ratio over 1.6; overall mean in the length/width ratio = 1.83 +/- 0.48) when bound to electron microscope specimen grids at low ionic strength (1mM EDTA, 10mM Tris, pH 8.0). A marked proportion of elongated core particles is also observed in the naturally occurring hyperacetylated chicken testis chromatin undergoing spermatogenesis when analyzed at low ionic strength (36.8% of the particles have a length/width ratio over 1.6). Core particles of elongated shape (length/width ratio over 1.6) generated under low ionic strength conditions are absent in the hypoacetylated chicken erythrocyte chromatin and represent only 2.3% of the untreated Hela S3 cell core particles containing a low proportion of hyperacetylated histones. The marked differences between control and hyperacetylated core particles are absent if the particles are bound to the carbon support film in the presence of 0.2 M NaCl, 6mM MgCl2 and 10mM Tris pH 8.0, conditions known to stabilize nucleosomes. A survey of the published work on histone hyperacetylation together with the present results indicate that histone hyperacetylation does not produce any marked disruption of the core particle 'per se', but that it decreases intranucleosomal stabilizing forces as judged by the lowered stability of the hyperacetylated core particle under conditions of shearing stress such as cationic competition by the carbon support film of the EM grid for DNA binding.
Full text
PDF
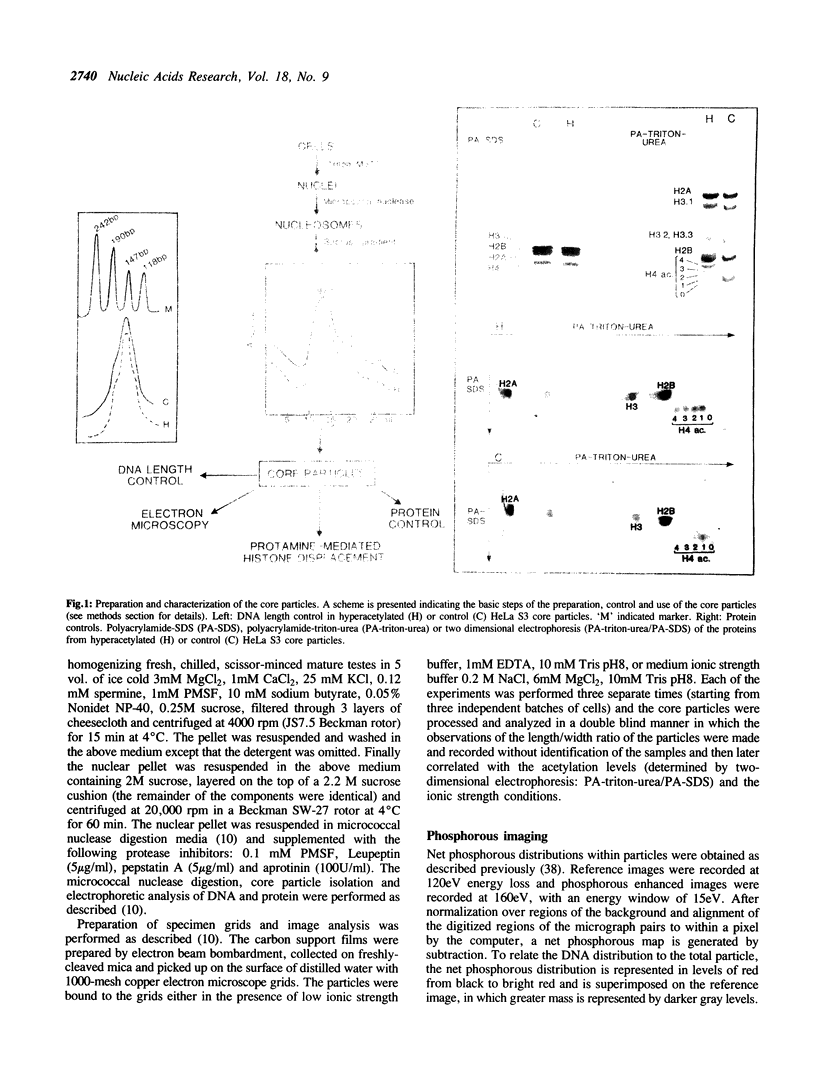

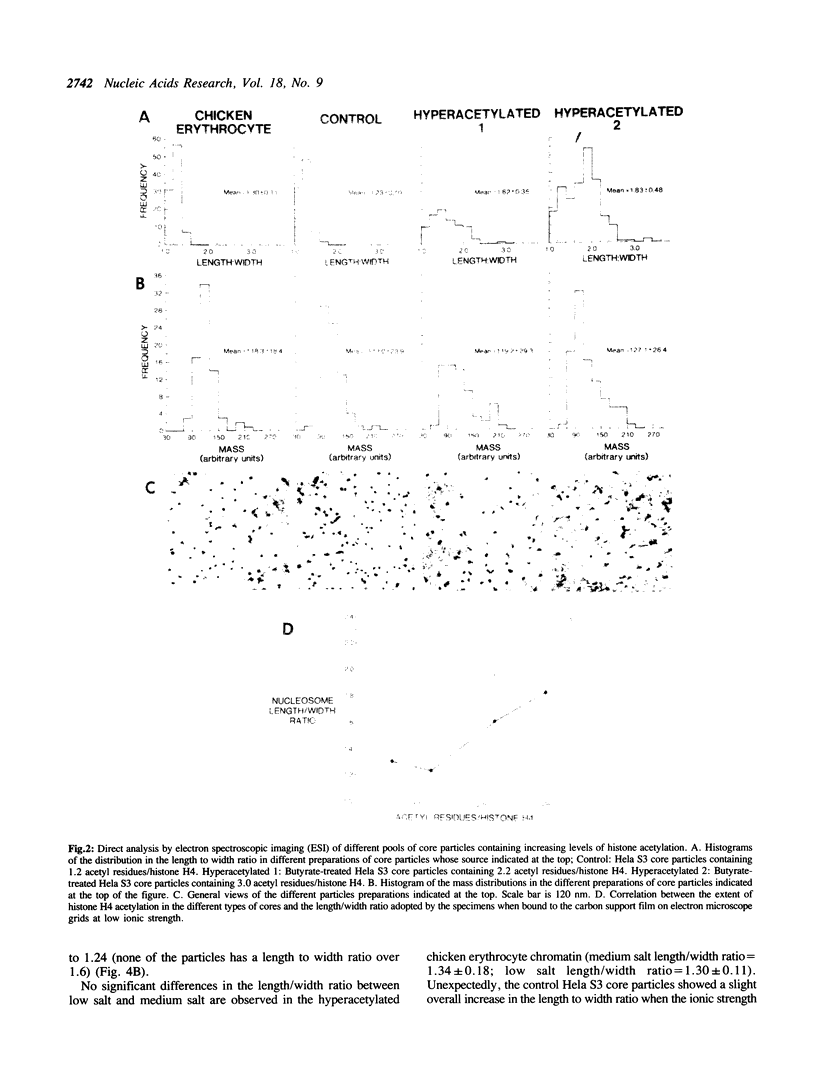
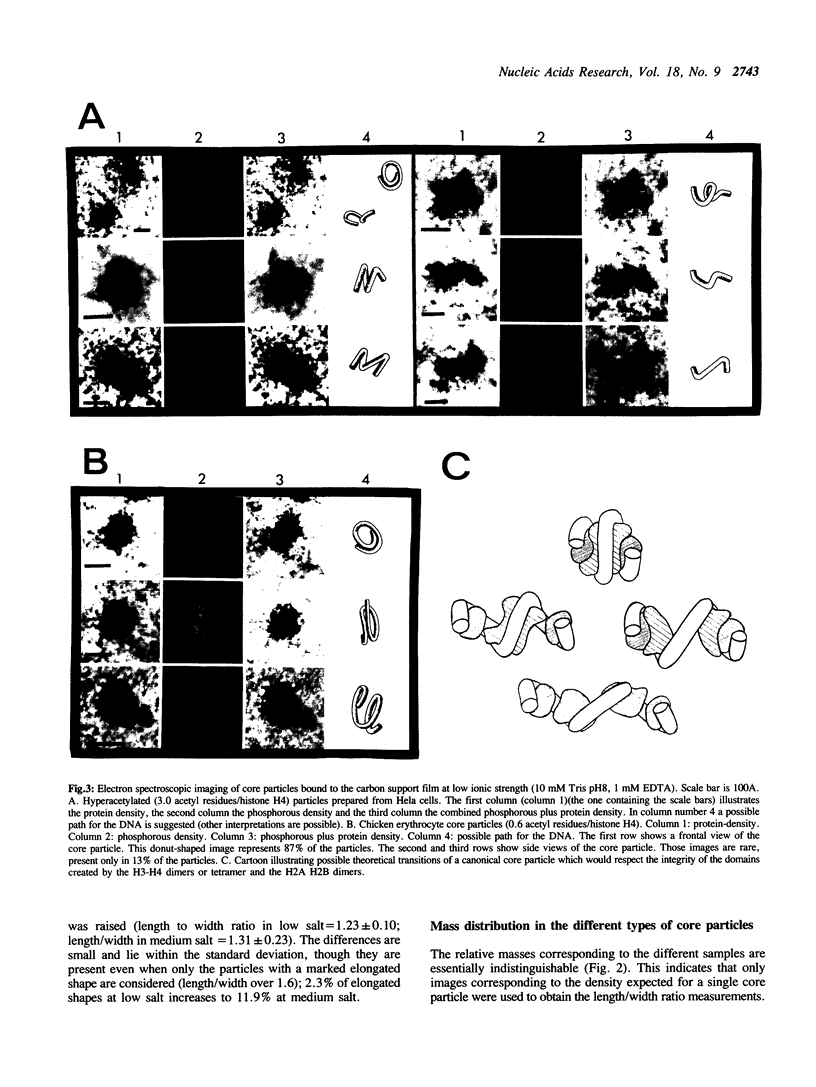
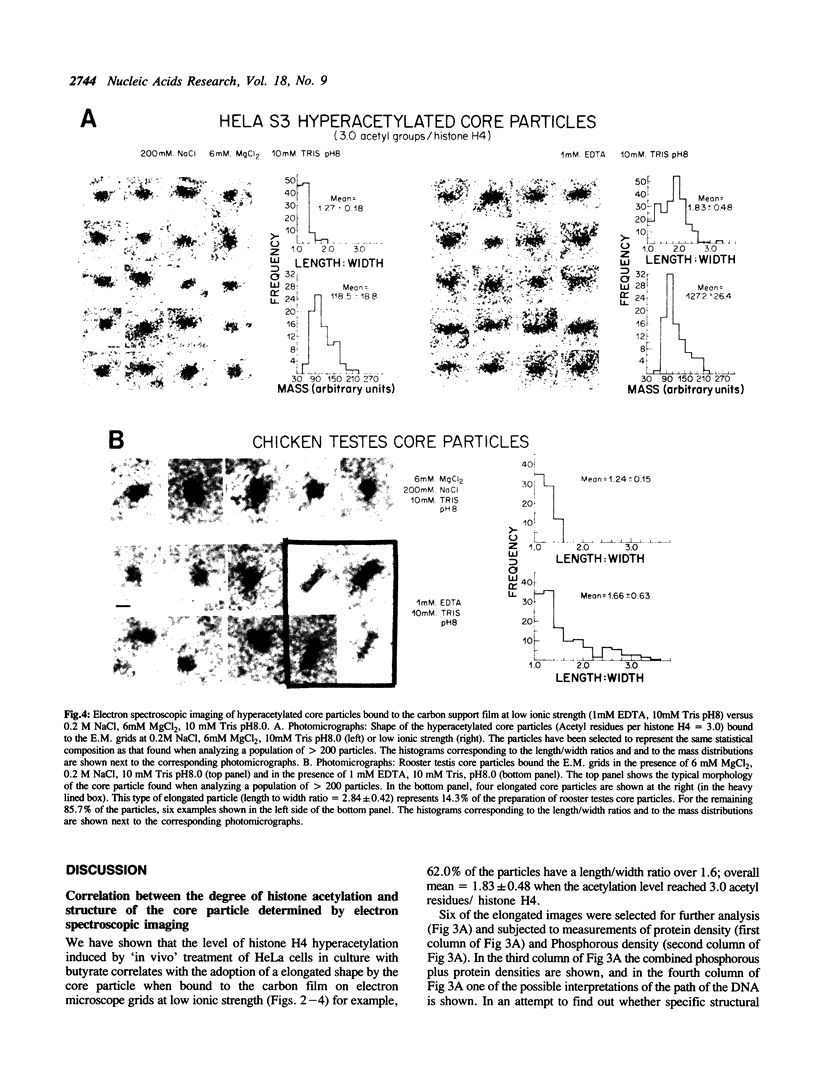



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra P., Sterner R., Clayton D. F., Allfrey V. G. Affinity chromatographic purification of nucleosomes containing transcriptionally active DNA sequences. J Mol Biol. 1987 Jul 20;196(2):379–388. doi: 10.1016/0022-2836(87)90698-x. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Chicoine L. G., Richman R., Schulman I. G. Deposition-related histone acetylation in micronuclei of conjugating Tetrahymena. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8048–8052. doi: 10.1073/pnas.82.23.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almer A., Rudolph H., Hinnen A., Hörz W. Removal of positioned nucleosomes from the yeast PHO5 promoter upon PHO5 induction releases additional upstream activating DNA elements. EMBO J. 1986 Oct;5(10):2689–2696. doi: 10.1002/j.1460-2075.1986.tb04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato A. T., Frado L. L., Seale R. L., Woodcock C. L. Treatment with sodium butyrate inhibits the complete condensation of interphase chromatin. Chromosoma. 1988;96(2):132–138. doi: 10.1007/BF00331045. [DOI] [PubMed] [Google Scholar]
- Ausio J., Seger D., Eisenberg H. Nucleosome core particle stability and conformational change. Effect of temperature, particle and NaCl concentrations, and crosslinking of histone H3 sulfhydryl groups. J Mol Biol. 1984 Jun 15;176(1):77–104. doi: 10.1016/0022-2836(84)90383-8. [DOI] [PubMed] [Google Scholar]
- Ausio J., van Holde K. E. Histone hyperacetylation: its effects on nucleosome conformation and stability. Biochemistry. 1986 Mar 25;25(6):1421–1428. doi: 10.1021/bi00354a035. [DOI] [PubMed] [Google Scholar]
- Balhorn R., Weston S., Thomas C., Wyrobek A. J. DNA packaging in mouse spermatids. Synthesis of protamine variants and four transition proteins. Exp Cell Res. 1984 Feb;150(2):298–308. doi: 10.1016/0014-4827(84)90572-x. [DOI] [PubMed] [Google Scholar]
- Bazett-Jones D. P., Brown M. L. Electron microscopy reveals that transcription factor TFIIIA bends 5S DNA. Mol Cell Biol. 1989 Jan;9(1):336–341. doi: 10.1128/mcb.9.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazett-Jones D. P., Locklear L., Rattner J. B. Electron spectroscopic imaging of DNA. J Ultrastruct Mol Struct Res. 1988 Apr;99(1):48–58. doi: 10.1016/0889-1605(88)90032-8. [DOI] [PubMed] [Google Scholar]
- Bertrand E., Erard M., Gómez-Lira M., Bode J. Influence of histone hyperacetylation on nucleosomal particles as visualized by electron microscopy. Arch Biochem Biophys. 1984 Feb 15;229(1):395–398. doi: 10.1016/0003-9861(84)90167-x. [DOI] [PubMed] [Google Scholar]
- Bode J., Gómez-Lira M. M., Schröter H. Nucleosomal particles open as the histone core becomes hyperacetylated. Eur J Biochem. 1983 Feb 15;130(3):437–445. doi: 10.1111/j.1432-1033.1983.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Bode J. Nucleosomal conformations induced by the small HMG proteins or by histone hyperacetylation are distinct. Arch Biochem Biophys. 1984 Jan;228(1):364–372. doi: 10.1016/0003-9861(84)90077-8. [DOI] [PubMed] [Google Scholar]
- Burlingame R. W., Love W. E., Wang B. C., Hamlin R., Nguyen H. X., Moudrianakis E. N. Crystallographic structure of the octameric histone core of the nucleosome at a resolution of 3.3 A. Science. 1985 May 3;228(4699):546–553. doi: 10.1126/science.3983639. [DOI] [PubMed] [Google Scholar]
- Candido E. P., Dixon G. H. Trout testis cells. 3. Acetylation of histones in different cell types from developing trout testis. J Biol Chem. 1972 Sep 10;247(17):5506–5510. [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Crane-Robinson C., Bradbury E. M., Dixon G. H. Effect of acetylation on the binding of N-terminal peptides of histone H4 to DNA. Eur J Biochem. 1982 Sep;127(1):137–143. doi: 10.1111/j.1432-1033.1982.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Chahal S. S., Matthews H. R., Bradbury E. M. Acetylation of histone H4 and its role in chromatin structure and function. Nature. 1980 Sep 4;287(5777):76–79. doi: 10.1038/287076a0. [DOI] [PubMed] [Google Scholar]
- Chambers S. A., Shaw B. R. Levels of histone H4 diacetylation decrease dramatically during sea urchin embryonic development and correlate with cell doubling rate. J Biol Chem. 1984 Nov 10;259(21):13458–13463. [PubMed] [Google Scholar]
- Chen T. A., Allfrey V. G. Rapid and reversible changes in nucleosome structure accompany the activation, repression, and superinduction of murine fibroblast protooncogenes c-fos and c-myc. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5252–5256. doi: 10.1073/pnas.84.15.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicoine L. G., Richman R., Cook R. G., Gorovsky M. A., Allis C. D. A single histone acetyltransferase from Tetrahymena macronuclei catalyzes deposition-related acetylation of free histones and transcription-related acetylation of nucleosomal histones. J Cell Biol. 1987 Jul;105(1):127–135. doi: 10.1083/jcb.105.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicoine L. G., Schulman I. G., Richman R., Cook R. G., Allis C. D. Nonrandom utilization of acetylation sites in histones isolated from Tetrahymena. Evidence for functionally distinct H4 acetylation sites. J Biol Chem. 1986 Jan 25;261(3):1071–1076. [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev Biol. 1982 Oct;93(2):404–415. doi: 10.1016/0012-1606(82)90127-0. [DOI] [PubMed] [Google Scholar]
- Christensen M. E., Rattner J. B., Dixon G. H. Hyperacetylation of histone H4 promotes chromatin decondensation prior to histone replacement by protamines during spermatogenesis in rainbow trout. Nucleic Acids Res. 1984 Jun 11;12(11):4575–4592. doi: 10.1093/nar/12.11.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. W., Hamkalo B. A. Nucleosome dissociation at physiological ionic strengths. Nucleic Acids Res. 1981 Jan 24;9(2):445–457. doi: 10.1093/nar/9.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couppez M., Martin-Ponthieu A., Sautière P. Histone H4 from cuttlefish testis is sequentially acetylated. Comparison with acetylation of calf thymus histone H4. J Biol Chem. 1987 Feb 25;262(6):2854–2860. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Carter S. P. Thermal stability of nucleosomes studied in situ by flow cytometry: effect of ionic strength and n-butyrate. Exp Cell Res. 1989 Feb;180(2):551–556. doi: 10.1016/0014-4827(89)90082-7. [DOI] [PubMed] [Google Scholar]
- Davie J. R., Candido E. P. Acetylated histone H4 is preferentially associated with template-active chromatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3574–3577. doi: 10.1073/pnas.75.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie J. R., Saunders C. A., Walsh J. M., Weber S. C. Histone modifications in the yeast S. Cerevisiae. Nucleic Acids Res. 1981 Jul 10;9(13):3205–3216. doi: 10.1093/nar/9.13.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D. K., Burkholder G. D. The effects of sodium and magnesium-ion interactions on chromatin structure and solubility. Eur J Cell Biol. 1985 Mar;36(2):315–322. [PubMed] [Google Scholar]
- Doenecke D., Gallwitz D. Acetylation of histones in nucleosomes. Mol Cell Biochem. 1982 Apr 30;44(2):113–128. doi: 10.1007/BF00226895. [DOI] [PubMed] [Google Scholar]
- Dresler S. L. Stimulation of deoxyribonucleic acid excision repair in human fibroblasts pretreated with sodium butyrate. Biochemistry. 1985 Nov 19;24(24):6861–6869. doi: 10.1021/bi00345a019. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Russo E., de Cristini F., Graziosi G., Micali F., Crane-Robinson C. Histone modification in early and late Drosophila embryos. Biochem J. 1984 Mar 1;218(2):321–329. doi: 10.1042/bj2180321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryev S. A., Krasheninnikov I. A. Transient unfolding of trypsin-digested chromatin core particles. Eur J Biochem. 1982 Dec;129(1):119–125. doi: 10.1111/j.1432-1033.1982.tb07029.x. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Henderson N. Acetylation of histones during spermatogenesis in the rat. Arch Biochem Biophys. 1983 Feb 15;221(1):108–116. doi: 10.1016/0003-9861(83)90126-1. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Henderson N. Acetylation of rat testis histones H2B and TH2B. Dev Biol. 1984 Feb;101(2):516–521. doi: 10.1016/0012-1606(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Grimes S. R., Jr, Henderson N. Hyperacetylation of histone H4 in rat testis spermatids. Exp Cell Res. 1984 May;152(1):91–97. doi: 10.1016/0014-4827(84)90232-5. [DOI] [PubMed] [Google Scholar]
- Hebbes T. R., Thorne A. W., Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988 May;7(5):1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M. Effects of histone acetylation on nucleosome properties as evaluated by polyacrylamide gel electrophoresis and hydroxylapatite dissociation chromatography. J Biochem. 1988 Jan;103(1):31–35. doi: 10.1093/oxfordjournals.jbchem.a122234. [DOI] [PubMed] [Google Scholar]
- Hirose M., Sarui K., Sunagawa A. Correlation between nuclear histone acetylation and casein messenger RNA induction in the mammary gland. J Biochem. 1985 Mar;97(3):781–789. doi: 10.1093/oxfordjournals.jbchem.a135118. [DOI] [PubMed] [Google Scholar]
- Huang S. Y., Garrard W. T. Temperature-dependent cleavage of chromatin by micrococcal nuclease near the nucleosome center. FEBS Lett. 1986 Apr 7;199(1):89–91. doi: 10.1016/0014-5793(86)81229-7. [DOI] [PubMed] [Google Scholar]
- Imai B. S., Yau P., Baldwin J. P., Ibel K., May R. P., Bradbury E. M. Hyperacetylation of core histones does not cause unfolding of nucleosomes. Neutron scatter data accords with disc shape of the nucleosome. J Biol Chem. 1986 Jul 5;261(19):8784–8792. [PubMed] [Google Scholar]
- Ip Y. T., Jackson V., Meier J., Chalkley R. The separation of transcriptionally engaged genes. J Biol Chem. 1988 Oct 5;263(28):14044–14052. [PubMed] [Google Scholar]
- Jackson V., Shires A., Tanphaichitr N., Chalkley R. Modifications to histones immediately after synthesis. J Mol Biol. 1976 Jun 25;104(2):471–483. doi: 10.1016/0022-2836(76)90282-5. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Sterner R., Allfrey V. G. Altered nucleosomes of active nucleolar chromatin contain accessible histone H3 in its hyperacetylated forms. J Biol Chem. 1987 May 25;262(15):6943–6946. [PubMed] [Google Scholar]
- Kadura S. N., Khrapunov S. N. Displacement of histones by sperm-specific proteins at different stages of spermatogenesis of squid. Eur J Biochem. 1988 Aug 15;175(3):603–607. doi: 10.1111/j.1432-1033.1988.tb14234.x. [DOI] [PubMed] [Google Scholar]
- Kayne P. S., Kim U. J., Han M., Mullen J. R., Yoshizaki F., Grunstein M. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell. 1988 Oct 7;55(1):27–39. doi: 10.1016/0092-8674(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Klug A., Rhodes D., Smith J., Finch J. T., Thomas J. O. A low resolution structure for the histone core of the nucleosome. Nature. 1980 Oct 9;287(5782):509–516. doi: 10.1038/287509a0. [DOI] [PubMed] [Google Scholar]
- Lewis P. N., Guillemette J. G., Chan S. Histone accessibility determined by lysine-specific acetylation in chicken erythrocyte nuclei. Eur J Biochem. 1988 Feb 15;172(1):135–145. doi: 10.1111/j.1432-1033.1988.tb13865.x. [DOI] [PubMed] [Google Scholar]
- Libertini L. J., Ausió J., van Holde K. E., Small E. W. Histone hyperacetylation. Its effects on nucleosome core particle transitions. Biophys J. 1988 Apr;53(4):477–487. doi: 10.1016/S0006-3495(88)83126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libertini L. J., Small E. W. Effects of pH on the stability of chromatin core particles. Nucleic Acids Res. 1984 May 25;12(10):4351–4359. doi: 10.1093/nar/12.10.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Leone J. W., Cook R. G., Allis C. D. Antibodies specific to acetylated histones document the existence of deposition- and transcription-related histone acetylation in Tetrahymena. J Cell Biol. 1989 May;108(5):1577–1588. doi: 10.1083/jcb.108.5.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D., Hereford L. Yeast chromatin is uniformly digested by DNase-I. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4285–4288. doi: 10.1073/pnas.76.9.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P., Gröbner P. Biosynthesis and posttranslational acetylation of histones during spherulation of Physarum polycephalum. Nucleic Acids Res. 1986 May 12;14(9):3745–3762. doi: 10.1093/nar/14.9.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P., Gröbner P. Postsynthetic acetylation of histones during the cell cycle: a general function for the displacement of histones during chromatin rearrangements. Nucleic Acids Res. 1987 Oct 26;15(20):8351–8366. doi: 10.1093/nar/15.20.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P., Loidl A., Puschendorf B., Gröbner P. RNA polymerase activity and template activity of chromatin after butyrate induced hyperacetylation of histones in Physarum. Nucleic Acids Res. 1984 Jul 11;12(13):5405–5417. doi: 10.1093/nar/12.13.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loidl P. Towards an understanding of the biological function of histone acetylation. FEBS Lett. 1988 Jan 25;227(2):91–95. doi: 10.1016/0014-5793(88)80874-3. [DOI] [PubMed] [Google Scholar]
- Lorch Y., LaPointe J. W., Kornberg R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell. 1987 Apr 24;49(2):203–210. doi: 10.1016/0092-8674(87)90561-7. [DOI] [PubMed] [Google Scholar]
- Louie A. J., Dixon G. H. Trout testis cells. II. Synthesis and phosphorylation of histones and protamines in different cell types. J Biol Chem. 1972 Sep 10;247(17):5498–5505. [PubMed] [Google Scholar]
- Makarov V. L., Smirnov I., Dimitrov S. I. Higher order folding of chromatin is induced in different ways by monovalent and by bivalent cations. FEBS Lett. 1987 Feb 23;212(2):263–266. doi: 10.1016/0014-5793(87)81357-1. [DOI] [PubMed] [Google Scholar]
- Marushige Y., Marushige K. Proteolysis of somatic type histones in transforming rat spermatid chromatin. Biochim Biophys Acta. 1983 Nov 22;761(1):48–57. doi: 10.1016/0304-4165(83)90361-6. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Nickol J. M., Felsenfeld G., Rau D. C. Histone hyperacetylation has little effect on the higher order folding of chromatin. Nucleic Acids Res. 1983 Jun 25;11(12):4065–4075. doi: 10.1093/nar/11.12.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. A., Perry W. M., Chalkley R. Sensitivity of regions of chromatin containing hyperacetylated histones to DNAse I. Biochem Biophys Res Commun. 1978 May 15;82(1):365–363. doi: 10.1016/0006-291x(78)90617-4. [DOI] [PubMed] [Google Scholar]
- Norton V. G., Imai B. S., Yau P., Bradbury E. M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989 May 5;57(3):449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- Oliva R., Bazett-Jones D., Mezquita C., Dixon G. H. Factors affecting nucleosome disassembly by protamines in vitro. Histone hyperacetylation and chromatin structure, time dependence, and the size of the sperm nuclear proteins. J Biol Chem. 1987 Dec 15;262(35):17016–17025. [PubMed] [Google Scholar]
- Oliva R., Dixon G. H. Chicken protamine genes are intronless. The complete genomic sequence and organization of the two loci. J Biol Chem. 1989 Jul 25;264(21):12472–12481. [PubMed] [Google Scholar]
- Oliva R., Goren R., Dixon G. H. Quail (Coturnix japonica) protamine, full-length cDNA sequence, and the function and evolution of vertebrate protamines. J Biol Chem. 1989 Oct 25;264(30):17627–17630. [PubMed] [Google Scholar]
- Oliva R., Mezquita C. Histone H4 hyperacetylation and rapid turnover of its acetyl groups in transcriptionally inactive rooster testis spermatids. Nucleic Acids Res. 1982 Dec 20;10(24):8049–8059. doi: 10.1093/nar/10.24.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva R., Mezquita C. Marked differences in the ability of distinct protamines to disassemble nucleosomal core particles in vitro. Biochemistry. 1986 Oct 21;25(21):6508–6511. doi: 10.1021/bi00369a025. [DOI] [PubMed] [Google Scholar]
- Oliva R., Mezquita J., Mezquita C., Dixon G. H. Haploid expression of the rooster protamine mRNA in the postmeiotic stages of spermatogenesis. Dev Biol. 1988 Feb;125(2):332–340. doi: 10.1016/0012-1606(88)90216-3. [DOI] [PubMed] [Google Scholar]
- Oliva R., Vidal S., Mezquita C. Cellular content and biosynthesis of polyamines during rooster spermatogenesis. Biochem J. 1982 Nov 15;208(2):269–273. doi: 10.1042/bj2080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T., Wrange O. Specific glucocorticoid receptor binding to DNA reconstituted in a nucleosome. EMBO J. 1988 Oct;7(10):3073–3079. doi: 10.1002/j.1460-2075.1988.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer U., Ferrari N., Tosetti F., Vidali G. Histone hyperacetylation is induced in chick erythrocyte nuclei during reactivation in heterokaryons. Exp Cell Res. 1988 Sep;178(1):25–30. doi: 10.1016/0014-4827(88)90374-6. [DOI] [PubMed] [Google Scholar]
- Pfeffer U., Ferrari N., Vidali G. Availability of hyperacetylated H4 histone in intact nucleosomes to specific antibodies. J Biol Chem. 1986 Feb 25;261(6):2496–2498. [PubMed] [Google Scholar]
- Piña B., Martínez P., Suau P. Differential acetylation of core histones in rat cerebral cortex neurons during development and aging. Eur J Biochem. 1988 Jun 1;174(2):311–315. doi: 10.1111/j.1432-1033.1988.tb14099.x. [DOI] [PubMed] [Google Scholar]
- Prior C. P., Cantor C. R., Johnson E. M., Littau V. C., Allfrey V. G. Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell. 1983 Oct;34(3):1033–1042. doi: 10.1016/0092-8674(83)90561-5. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Ramanathan B., Smerdon M. J. Enhanced DNA repair synthesis in hyperacetylated nucleosomes. J Biol Chem. 1989 Jul 5;264(19):11026–11034. [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Richman R., Chicoine L. G., Collini M. P., Cook R. G., Allis C. D. Micronuclei and the cytoplasm of growing Tetrahymena contain a histone acetylase activity which is highly specific for free histone H4. J Cell Biol. 1988 Apr;106(4):1017–1026. doi: 10.1083/jcb.106.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Roca J., Mezquita C. DNA topoisomerase II activity in nonreplicating, transcriptionally inactive, chicken late spermatids. EMBO J. 1989 Jun;8(6):1855–1860. doi: 10.1002/j.1460-2075.1989.tb03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Schröter H., Gómez-Lira M. M., Plank K. H., Bode J. The extent of histone acetylation induced by butyrate and the turnover of acetyl groups depend on the nature of the cell line. Eur J Biochem. 1981 Nov;120(1):21–28. doi: 10.1111/j.1432-1033.1981.tb05664.x. [DOI] [PubMed] [Google Scholar]
- Singh J., Rao M. R. Interaction of rat testis protein, TP, with nucleosome core particle. Biochem Int. 1988 Oct;17(4):701–710. [PubMed] [Google Scholar]
- Sterner R., Boffa L. C., Chen T. A., Allfrey V. G. Cell cycle-dependent changes in conformation and composition of nucleosomes containing human histone gene sequences. Nucleic Acids Res. 1987 Jun 11;15(11):4375–4391. doi: 10.1093/nar/15.11.4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Fellows G. Specific antibodies reveal ordered and cell-cycle-related use of histone-H4 acetylation sites in mammalian cells. Eur J Biochem. 1989 Jan 15;179(1):131–139. doi: 10.1111/j.1432-1033.1989.tb14530.x. [DOI] [PubMed] [Google Scholar]
- Waterborg J. H., Matthews H. R. Intranuclear localization of histone acetylation in Physarum polycephalum and the structure of functionally active chromatin. Cell Biophys. 1983 Dec;5(4):265–279. doi: 10.1007/BF02788625. [DOI] [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Supercoiling facilitates lac operator-repressor-pseudooperator interactions. J Biol Chem. 1987 Apr 15;262(11):4943–4946. [PubMed] [Google Scholar]
- Zhang D. E., Nelson D. A. Histone acetylation in chicken erythrocytes. Rates of acetylation and evidence that histones in both active and potentially active chromatin are rapidly modified. Biochem J. 1988 Feb 15;250(1):233–240. doi: 10.1042/bj2500233. [DOI] [PMC free article] [PubMed] [Google Scholar]






