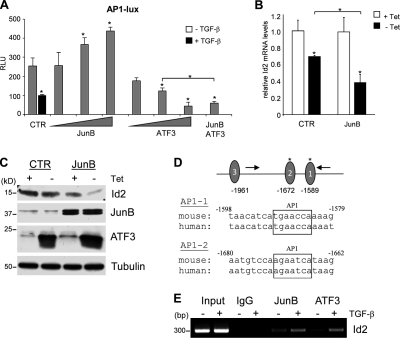
Figure 7.
JunB and ATF3 cooperate to mediate transcriptional repression of Id2 by TGF-β. (A) NMuMG cells were cotransfected with AP1-Lux luciferase reporter, a Renilla luciferase reporter under the control of the herpes simplex virus thymidine kinase promoter, and increasing amounts of JunB and ATF3 (1, 2, and 4 µg) or both factors together (2 µg each). Cells were treated with 2 ng/ml TGF-β1 for 16 h where indicated. CTR, control; RLU, relative luciferase unit. Experiments were performed in triplicate and repeated at least twice. Error bars represent the mean value ± SD. (B and C) NMuMG–Tet-Off–ATF3 control or JunB-overexpressing cells were grown in the absence or presence of 1 µg/ml tetracycline for 24 h followed by treatment with 2 ng/ml TGF-β1 for 24 h. Total RNA and whole-cell extracts were used for quantitative RT-PCR (B) and immunoblotting (C). (B) Quantitative PCR for Id2 was performed in triplicate. The CT values were normalized to 18S rRNA and presented as fold difference relative to untreated control. Error bars represent the mean value ± SD. (C) Immunoblotting of Id2, JunB, ATF3, and tubulin, a loading control. (D) Location of conserved AP1 sites within the mouse Id2 promoter relative to primers used for ChIP studies. Boxed regions indicate the putative binding sites for AP1 transcription factor. (A, B, and D) *, P < 0.05 using a Student’s t test. (E) ChIP experiments were performed with NMuMG cells after treatment with 2 ng/ml TGF-β1 for 8 h. Amplifications were performed using DNA samples before precipitation (Input) and after precipitation with control IgG or antibodies to JunB or ATF3.