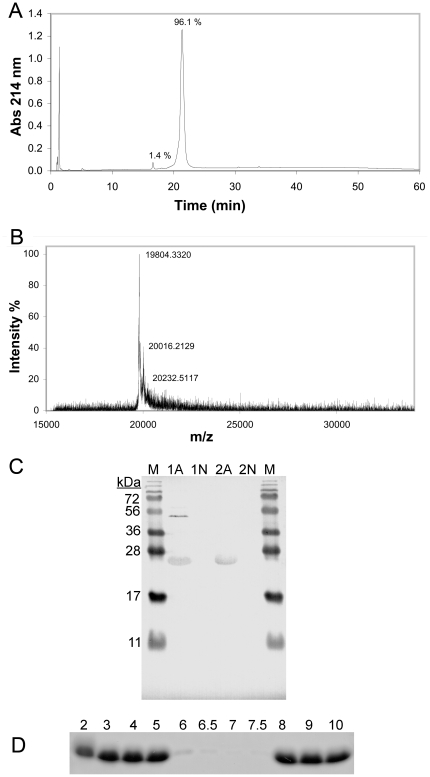
Figure 2. Characterization of recombinant human amelogenin purified using an acid/heat treatment method.
A) C18 reverse phase HPLC analysis of amelogenin purified with the acid/heat treatment method. Amelogenin elutes at ∼45% acetonitrile and the peak area indicates a purity of >95%. B) MALDI-TOF mass spectra of purified amelogenin recorded in linear mode. The obtained mass is very close from expected theoretical mass (19804.8 Da). The peaks seen with slightly higher masses than the main peak is most likely satellite signals derived from matrix adducts that have reacted with the polypeptide. C) Western blot of amelogenin samples (A) and negative controls (N). Lanes marked 1 are samples taken from cultivated cells, and lanes marked 2 are from samples after the acid/heat treatment. Lanes marked M contain molecular weight markers. The amelogenin containing samples show a positive reaction not seen in the negative controls, confirming the identity of the protein. The band visible at higher molecular weights in lane 1A suggests that a multimeric form of amelogenin is present inside the cells. D) SDS-PAGE analysis of amelogenin solubility test, showing the soluble fractions of purified amelogenin at pH 2–10. The protein is only sparingly soluble at pH 6–7.5, but is readily soluble at more acidic or basic pH.