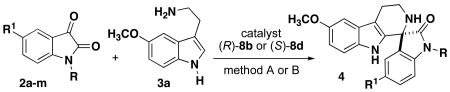
Table 2. Scope of Isatins for Phosphoric acid-catalyzed Spirocyclization.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | R1 | product | method | yield (%)[a] | er[b] |
| 1 | CH3 | Br | 4aa | A | 99 | 91:9 |
| B | 70 | 82:18 | ||||
| 2 | CH3 | Cl | 4ba | A | 99 | 92:8 |
| B | 90 | 88:12 | ||||
| 3 | CH3 | H | 4ca | A | 99 | 88:12 |
| B | 99 | 95:5 | ||||
| 4 | CH3 | OCH3 | 4da | A | 99 | 58:42 |
| B | 79 | 70:30 | ||||
| 5 | Bn | H | 4ea | A | 99 | 82:18 |
| B | 95 | 97:3 | ||||
| 6 | PMB | H | 4fa | A | 72 | 87:13 |
| B | 87 | 93:7 | ||||
| 7 | PMB | Br | 4ga | A | 86 | 87:13 |
| B | 81 | 75:25 | ||||
| 8 | CH2CCH | F | 4ha | A | 60 | 92:8 |
| B | 86 | 92:8 | ||||
| 9 | Ph | H | 4ia | A | 99 | 85:15 |
| B | 44 | 97:3 | ||||
| 10 | Ac | H | 4ja | A | 87[c] | -- |
| B | 53[c] | -- | ||||
| 11 | H | H | 4ka | A | 66 | 94:6 |
| B | 99 | 94:6 | ||||
| 12 | H | Cl | 4la | A | 99 | 84:16 |
| B | 88 | 80:20 | ||||
| 13 | H | OCH3 | 4ma | A | 84 | 97:3 |
| B | 92 | 96:4 | ||||
Method A: 20 mol % catalyst (R)-8b in CH2Cl2 at 23°C for 72-96h.
Method B: 10 mol % catalyst (S)-8d in DMF at 40°C for 24-48h.
Isolated yield.
Enantioselectivity determined by chiral phase HPLC.
Yield is for imine.
